Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities - PubMed (original) (raw)
doi: 10.1186/1471-2407-8-17.
Alessandro De Luca, Niki Boyd, Sean Young, Armelle Troussard, Yolanda Ridge, Pardeep Kaurah, Steve E Kalloger, Katherine A Blood, Margaret Smith, Paul T Spellman, Yuker Wang, Dianne M Miller, Doug Horsman, Malek Faham, C Blake Gilks, Joe Gray, David G Huntsman
Affiliations
- PMID: 18208621
- PMCID: PMC2245962
- DOI: 10.1186/1471-2407-8-17
Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities
Joshua Z Press et al. BMC Cancer. 2008.
Abstract
Background: Subclassification of ovarian carcinomas can be used to guide treatment and determine prognosis. Germline and somatic mutations, loss of heterozygosity (LOH), and epigenetic events such as promoter hypermethylation can lead to decreased expression of BRCA1/2 in ovarian cancers. The mechanism of BRCA1/2 loss is a potential method of subclassifying high grade serous carcinomas.
Methods: A consecutive series of 49 ovarian cancers was assessed for mutations status of BRCA1 and BRCA2, LOH at the BRCA1 and BRCA2 loci, methylation of the BRCA1 promoter, BRCA1, BRCA2, PTEN, and PIK3CA transcript levels, PIK3CA gene copy number, and BRCA1, p21, p53, and WT-1 immunohistochemistry.
Results: Eighteen (37%) of the ovarian carcinomas had germline or somatic BRCA1 mutations, or epigenetic loss of BRCA1. All of these tumours were high-grade serous or undifferentiated type. None of the endometrioid (n = 5), clear cell (n = 4), or low grade serous (n = 2) carcinomas showed loss of BRCA1, whereas 47% of the 38 high-grade serous or undifferentiated carcinomas had loss of BRCA1. It was possible to distinguish high grade serous carcinomas with BRCA1 mutations from those with epigenetic BRCA1 loss: tumours with BRCA1 mutations typically had decreased PTEN mRNA levels while those with epigenetic loss of BRCA1 had copy number gain of PIK3CA. Overexpression of p53 with loss of p21 expression occurred significantly more frequently in high grade serous carcinomas with epigenetic loss of BRCA1, compared to high grade serous tumors without loss of BRCA1.
Conclusion: High grade serous carcinomas can be subclassified into three groups: BRCA1 loss (genetic), BRCA1 loss (epigenetic), and no BRCA1 loss. Tumors in these groups show distinct molecular alterations involving the PI3K/AKT and p53 pathways.
Figures
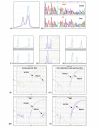
Figure 1
Assessment of BRCA1 loss (A) Mutation screening showing the abnormal denaturing high performance liquid chromatography profile corresponding to the 1351delAT mutation in tumor 223. The single blue line represents the electropherogram from a normal control, while the purple line represents the abnormal profile formed by the mutated exon 11c in tumor 223. (B) Direct DNA sequencing demonstrating the 185delAG mutation in tumor 283. Only the mutant allele is seen in the tumor because LOH is present. (C-E) Loss of heterozygosity (LOH) analysis using BRCA1-associated microsatellite markers visualized on an ABI Prism 3100 Genetic Analyzer, where LOH is defined as >50% decrease in area under the curve when germline DNA (upper tracing) and tumor DNA (lower tracing) are compared. (C) The lack of LOH in tumor 240 demonstrated using microsatellite marker D17S1185, (D) LOH in tumor 283 demonstrated using microsatellite marker D17S855. (E) Microsatellite instability demonstrated in tumor 156 using microsatellite marker D17S1185. (F, G, H, and I) Methylation analysis of BRCA1 gene using fluorescence-based, quantitative, real-time PCR (TaqMan) using SYBR Green 1 as detection method. Two sets of primers, designed specifically for bisulfite converted DNA, were used: a methylated set for the BRCA1 gene and a reference set (MYOD1) to control for input DNA. Specificity of the reactions for methylated DNA were confirmed separately using human genomic DNA (unmethyated; F) and CpG methylated Jurkat genomic DNA (methylated; G), respectively. H and I show representative examples of results from assessment of BRCA1 loss through promoter hypermethyation. Tumor 178 shows only unmethylated BRCA1 promoter, while tumor 345 shows evidence of BRCA1 promoter hypermethylation.
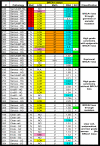
Figure 2
Summary of BRCA1 abnormalities and associated features: Pathology refers to the tumor histopathology. Serous or Ser = serous carcinoma; Undiff = undifferentiated carcinoma; HG = high-grade; LG = low-grade; Clear cell = clear cell carcinoma; Endo = endometrioid carcinoma; G1 = grade 1; G2 = grade 2; G3 = grade 3. BRCA1 Status: Mut = mutation; G = germline; S = somatic; N = no mutations. LOH = loss of heterozygosity where LOH indicates that loss of heterozygosity is present, NO indicates that loss of heterozygosity is not present, and MSI indicates that microsatellite instability is present in the tumor. Meth refers to BRCA1 promoter hypermethylation. Tumors containing ≥ 4% fully methylated molecules are designated as methylated (M) and are highlighted in orange, whereas tumors containing < 4% are designated as unmethylated (U). RNA refers to relative RNA expression compared to the average RNA expression in all samples, where the average RNA expression = 1.0. Tumors with relative RNA expression <0.7 are highlighted in aqua as showing BRCA1/BRCA2 loss. IHC refers to BRCA1 immunohistochemistry; (+) indicates tumors with > 1% of nuclei stained positive for BRCA1, (-) indicates tumors with <1% of nuclei positive. N/A indicates that the data is not available for technical reasons.
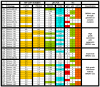
Figure 3
Summary of analysis of high grade (HG) serous/undifferentiated ovarian tumors: MIP copy number results are shown for c-myc and PIK3CA loci. MIP copy number values over 3.0 are highlighted and correspond to amplification. Relative mRNA levels for PIK3CA and PTEN were assessed using qRT-PCR; levels over 1.3 (highlighted in green) are considered elevated and levels below 0.7 (highlighted in aqua) indicate decreased transcript levels. Associated immunohistochemical markers p21, p53, and WT-1 refer to immunohistochemical staining results. Scoring of immunostaining was done as follows: p21: 0 = <5% nuclei positive and 1 = >5% of nuclei positive. p53: 0 = <50% nuclei positive and 1 = >50% of nuclei positive. WT1: 0 = <5% nuclei positive, 1 = 5–50% nuclei positive, and 2 = >50% nuclei positive. N/A indicates that the data is not available for technical reasons.
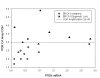
Figure 4
Correlation between decreased PTEN mRNA levels and amplification at the PIK3CA locus: Relative PTEN mRNA levels as determined by qRT-PCR are plotted along the X-axis and PIK3CA MIP copy number results are plotted along the Y-axis for high grade serous ovarian tumors with BRCA1 mutations (open circles) and high grade serous ovarian tumors with epigenetic loss of BRCA1 (filled triangles). MIP copy number values over 3.0 indicate amplification.
Figure 5
Immunohistochemistry results: Representative immunohistochemistry results for high grade serous ovarian tumors with BRCA1 mutations (tumor #327, top row), with epigenetic loss of BRCA1 (tumor #332, middle row), and without loss of BRCA1 (tumor #372, bottom row). Immunohistochemical staining is shown for BRCA1 (left column), p53 (middle column) and p21 (right column).
Similar articles
- BRCA1 immunohistochemistry in a molecularly characterized cohort of ovarian high-grade serous carcinomas.
Garg K, Levine DA, Olvera N, Dao F, Bisogna M, Secord AA, Berchuck A, Cerami E, Schultz N, Soslow RA. Garg K, et al. Am J Surg Pathol. 2013 Jan;37(1):138-46. doi: 10.1097/PAS.0b013e31826cabbd. Am J Surg Pathol. 2013. PMID: 23232854 Free PMC article. - Gross genomic alterations and gene expression profiles of high- grade serous carcinoma of the ovary with and without BRCA1 inactivation.
Pradhan M, Risberg BA, Tropé CG, van de Rijn M, Gilks CB, Lee CH. Pradhan M, et al. BMC Cancer. 2010 Sep 15;10:493. doi: 10.1186/1471-2407-10-493. BMC Cancer. 2010. PMID: 20843305 Free PMC article. - BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma.
McAlpine JN, Porter H, Köbel M, Nelson BH, Prentice LM, Kalloger SE, Senz J, Milne K, Ding J, Shah SP, Huntsman DG, Gilks CB. McAlpine JN, et al. Mod Pathol. 2012 May;25(5):740-50. doi: 10.1038/modpathol.2011.211. Epub 2012 Jan 27. Mod Pathol. 2012. PMID: 22282309 - Origins and molecular pathology of ovarian cancer.
Bell DA. Bell DA. Mod Pathol. 2005 Feb;18 Suppl 2:S19-32. doi: 10.1038/modpathol.3800306. Mod Pathol. 2005. PMID: 15761464 Review. - Molecular pathology of epithelial ovarian cancer.
Christie M, Oehler MK. Christie M, et al. J Br Menopause Soc. 2006 Jun;12(2):57-63. doi: 10.1258/136218006777525794. J Br Menopause Soc. 2006. PMID: 16776856 Review.
Cited by
- Poly(Adenosine diphosphate-ribose) polymerase inhibitors in cancer treatment.
Park SR, Chen A. Park SR, et al. Hematol Oncol Clin North Am. 2012 Jun;26(3):649-70, ix. doi: 10.1016/j.hoc.2012.02.012. Hematol Oncol Clin North Am. 2012. PMID: 22520984 Free PMC article. Review. - Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy.
Romero I, Bast RC Jr. Romero I, et al. Endocrinology. 2012 Apr;153(4):1593-602. doi: 10.1210/en.2011-2123. Epub 2012 Mar 13. Endocrinology. 2012. PMID: 22416079 Free PMC article. Review. - Loss of heterozygosity at BRCA1/2 loci in hereditary and sporadic ovarian cancers.
Brozek I, Ochman K, Debniak J, Morzuch L, Ratajska M, Stepnowska M, Stukan M, Emerich J, Limon J. Brozek I, et al. J Appl Genet. 2009;50(4):379-84. doi: 10.1007/BF03195697. J Appl Genet. 2009. PMID: 19875889 - Molecular abnormalities in ovarian cancer subtypes other than high-grade serous carcinoma.
Gilks CB. Gilks CB. J Oncol. 2010;2010:740968. doi: 10.1155/2010/740968. Epub 2009 Dec 30. J Oncol. 2010. PMID: 20069115 Free PMC article.
References
- Milner BJ, Allan LA, Eccles DM, Kitchener HC, Leonard RC, Kelly KF, Parkin DE, Haites NE. p53 mutation is a common genetic event in ovarian carcinoma. Cancer Res. 1993;53:2128–2132. - PubMed
- Soslow RA, Shen PU, Chung MH, Isacson C. Distinctive p53 and mdm2 immunohistochemical expression profiles suggest different pathogenetic pathways in poorly differentiated endometrial carcinoma. Int J Gynecol Pathol. 1998;17:129–134. - PubMed
- Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, Shih Ie M. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA83639/CA/NCI NIH HHS/United States
- P01 CA064602/CA/NCI NIH HHS/United States
- P01 CA 64602/CA/NCI NIH HHS/United States
- P50 CA 58207/CA/NCI NIH HHS/United States
- P50 CA058207/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous