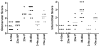Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis - PubMed (original) (raw)
Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis
Lena Schiffer et al. J Immunol. 2008.
Erratum in
- J Immunol. 2008 Mar 1;180(5):3613. Madaio, Michael M [corrected to Madaio, Michael P]
Abstract
Costimulatory blockade with CTLA4Ig and anti-CD40L along with a single dose of cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F(1) mice. To understand the mechanisms for remission and for impending relapse, we examined the expression profiles of 61 inflammatory molecules in the perfused kidneys of treated mice and untreated mice at different stages of disease. Further studies using flow cytometry and immunohistochemistry allowed us to determine the cellular origins of several key markers. We show that only a limited set of inflammatory mediators is expressed in the kidney following glomerular immune complex deposition but before the onset of proteinuria. Formation of a lymphoid aggregate in the renal pelvis precedes the invasion of the kidney by inflammatory cells. Regulatory molecules are expressed early in the disease process and during remission but do not prevent the inevitable progression of active inflammation. Onset of proliferative glomerulonephritis and proteinuria is associated with activation of the renal endothelium, expression of chemokines that mediate glomerular cell infiltration, and infiltration by activated dendritic cells and macrophages that migrate to different topographical areas of the kidney but express a similar profile of inflammatory cytokines. Increasing interstitial infiltration by macrophages and progressive tubular damage, manifested by production of lipocalin-2, occur later in the disease process. Studies of treated mice identify a type II (M2b)-activated macrophage as a marker of remission induction and impending relapse and suggest that therapy for systemic lupus erythematosus nephritis should include strategies that prevent both activation of monocytes and their migration to the kidney.
Figures
FIGURE 1
Histologic scores of H&E sections of kidneys examined by light microscopy. The six groups of mice used in this study are described in the text. Untreated mice are in black and treated mice in gray. A, Glomerular scores and interstitial scores (B). Each symbol indicates an individual mouse.
FIGURE 2
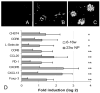
Early expression of inflammatory markers in NZB/W kidneys: upper panels, fluorescence for IgG at 16 wk (A), 23 wk before proteinuria onset (B), and 23 wk at onset of proteinuria (C). Data are representative of five mice examined in each group. D, Significantly up-regulated (>2-fold) genes by SAM and t test (mean + 1 SD) in the kidneys of 23-wk nonproteinuric mice (23w NP). Values are normalized to the mean of 6- to 16-wk mice given a value of 1. Data are shown as log 2. Values of p for the up-regulated genes are p < 0.01 (*) or p < 0.001 (**) as shown.
FIGURE 3
Genes up-regulated at the onset of proteinuria. Gene expression for age-matched mice with (23w P) and without (23w NP) proteinuria was normalized to the mean of 6- to 16-wk controls given a value of 1. Data are shown as log 2. Genes significantly up- or down-regulated (>2-fold) by SAM and t test (mean + 1 SD) are shown. Values of p for the up-regulated genes are p < 0.01 (*) or p < 0.001 (**) as shown.
FIGURE 4
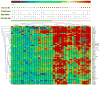
Two-way hierarchical cluster analysis of genes with significantly altered expression by ANOVA in the four groups of untreated mice color coded as follows: 6w, green; 16w, light blue; 23w NP, dark blue; 23w P, orange; and 36 – 40w, red. Proteinuria and renal glomerular and interstitial scores are shown for each mouse. Gray squares indicate assay not done. Nonproteinuric and proteinuric mice cluster separately. Within the nonproteinuric group, the older mice almost all cluster together and within the proteinuric group the mice with new onset proteinuria cluster together (yellow box).
FIGURE 5
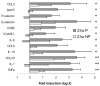
Altered renal gene expression after remission induction. A, The genes that were significantly altered 3– 4 wk after remission induction compared with mice with new onset proteinuria (23w P) and genes that changed between early remission (3– 4w post) and the later stages of remission in which relapse is impending (≥5w post). Values were normalized to the mean of young 6- to 16-wk mice given a value of 1. Data are shown as log 2. Genes significantly up- or down-regulated (>2-fold) by SAM and t test (mean + 1 SD) are shown. Values of p for the up-regulated genes are NS, p < 0.01 (*), or p < 0.001 (**) as shown. B, The individual results for genes significantly different by ANOVA analysis in the three groups are shown in the upper panel. Young mice are shown for comparison but were not included in the ANOVA analysis.
FIGURE 6
Flow cytometry analysis of renal-infiltrating cells: A–G, The upper plot shows spleen (A–E) or PBMC (F and G) and the lower plot shows kidney cells from the same mouse. Cells were gated on live cells and lymphocytes with further gates as shown above each set of plots. Renal CD4 T cells were activated and were similar in phenotype to those found in the spleen (A and B). Renal B cells included follicular (IgMintIgDhigh), immature (IgMhigh IgDlow), and class-switched (IgMlowIgDlow) cells (C), as well as B1cells (B220int CD5high) (D). Marginal zone B cells (CD23lowCD21high) were absent (E). Both peripheral blood and kidney CD11b- positive monocytes were predominantly negative for Ly6C, but kidney cells had also down-regulated CD43 (F and G), Kidney (gray histograms) and spleen cells (black histograms) from matched mice are compared in H–J. Kidney CD11b cells consisted of a major population of F4/80high macrophages (H) that had up-regulated CD80 and CD86 (I) and a smaller population of CD11chigh dendritic cells (J). Splenic CD11b-positive cells from the same mice contained a small population of F4/80-positive cells that had not up-regulated CD86 and a large population of CD11chigh dendritic cells. Renal CD11b cells from young (gray histograms) and nephritic mice (black histograms) are compared in K and L. CD11b cells from nephritic mice have higher expression of CD11b (K) and Ox-40L (L). Results are representative of samples from at least three mice examined in each group.
FIGURE 7
Immunohistochemical analysis of kidneys. A–F, Stained with PE-conjugated Abs as indicated and counterstained with 4′,6-diamidino-2-phenylindole (blue). A–C, F4/80high macrophages are scattered throughout the renal parenchyma and are found surrounding glomeruli and form a cap around perivascular and perihilar lymphoid aggregates. B cells and CD11chigh dendritic cells are found in the lymphoid aggregates, whereas CD4 T cells are found in aggregates and around glomeruli (D–F). Note the virtual disappearance of macrophages from the kidneys of mice in remission (G). H–K, Foxp3 staining of lymphoid aggregates from mice at different disease stages as marked. Foxp3-positive cells appeared in the hilar aggregates early in disease and did not disappear upon remission induction. L and M, Large numbers of plasma cells localized mainly in the hilar aggregates of a 36-wk proteinuric mouse. N and O, Double staining for CD4 and L-selectin (CD62L). L-selectin-positive T cells remained in the renal hilar aggregate after remission induction. Images are ×10 unless indicated otherwise. Images are representative of at least three mice examined in each group.
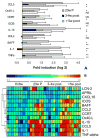
FIGURE 8
Cellular source of key markers: fold induction of the indicated inflammatory markers in sorted cells from the kidneys of nephritic mice shown as mean ± SEM. Total kidney cells and sorted lymphoid (Lym) and intrinsic renal cells (Int) were obtained from one group of five mice and CD4 T cells, CD19 B cells, macrophages, and dendritic cells were sorted from a second group as described in the text. Significant differences between groups are as indicated (*, p < 0.03; †, p < 0.01; ‡, p < 0.02; §, p < 0.001; ¶, p < 0.05).
Similar articles
- Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition.
Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, Madaio MP, Davidson A. Schiffer L, et al. J Immunol. 2003 Jul 1;171(1):489-97. doi: 10.4049/jimmunol.171.1.489. J Immunol. 2003. PMID: 12817034 - A unique hybrid renal mononuclear phagocyte activation phenotype in murine systemic lupus erythematosus nephritis.
Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y, Bottinger EP, Ivashkiv L, Kretzler M, Davidson A. Bethunaickan R, et al. J Immunol. 2011 Apr 15;186(8):4994-5003. doi: 10.4049/jimmunol.1003010. Epub 2011 Mar 16. J Immunol. 2011. PMID: 21411733 Free PMC article. - Laquinimod delays and suppresses nephritis in lupus-prone mice and affects both myeloid and lymphoid immune cells.
Lourenço EV, Wong M, Hahn BH, Palma-Diaz MF, Skaggs BJ. Lourenço EV, et al. Arthritis Rheumatol. 2014 Mar;66(3):674-85. doi: 10.1002/art.38259. Arthritis Rheumatol. 2014. PMID: 24574228 - Lupus nephritis: redefining the treatment goals.
De Vriese AS, Sethi S, Fervenza FC. De Vriese AS, et al. Kidney Int. 2025 Feb;107(2):198-211. doi: 10.1016/j.kint.2024.10.018. Epub 2024 Nov 8. Kidney Int. 2025. PMID: 39521057 Review. - Lupus nephritis: the evolving role of novel therapeutics.
Rovin BH, Parikh SV. Rovin BH, et al. Am J Kidney Dis. 2014 Apr;63(4):677-90. doi: 10.1053/j.ajkd.2013.11.023. Epub 2014 Jan 7. Am J Kidney Dis. 2014. PMID: 24411715 Free PMC article. Review.
Cited by
- The role of organ-deposited IgG in the pathogenesis of multi-organ and tissue damage in systemic lupus erythematosus.
Qiu W, Yu T, Deng GM. Qiu W, et al. Front Immunol. 2022 Oct 13;13:924766. doi: 10.3389/fimmu.2022.924766. eCollection 2022. Front Immunol. 2022. PMID: 36311714 Free PMC article. Review. - Disulfiram alleviates pristane-induced lupus via inhibiting GSDMD-mediated pyroptosis.
Zhuang L, Luo X, Wu S, Lin Z, Zhang Y, Zhai Z, Yang F, Li Y, Zhuang J, Luo G, Xu W, He Y, Sun E. Zhuang L, et al. Cell Death Discov. 2022 Sep 3;8(1):379. doi: 10.1038/s41420-022-01167-2. Cell Death Discov. 2022. PMID: 36057687 Free PMC article. - Pathogenesis of proliferative lupus nephritis from a historical and personal perspective.
Fu SM, Wang H, Dai C, Sung SJ, Gaskin F. Fu SM, et al. Clin Immunol. 2017 Dec;185:51-58. doi: 10.1016/j.clim.2016.07.024. Epub 2016 Aug 31. Clin Immunol. 2017. PMID: 27591148 Free PMC article. Review. No abstract available. - LMW heparin prevents increased kidney expression of proinflammatory mediators in (NZBxNZW)F1 mice.
Hedberg A, Kanapathippillai P, Rekvig OP, Fenton KA. Hedberg A, et al. Clin Dev Immunol. 2013;2013:791262. doi: 10.1155/2013/791262. Epub 2013 Sep 17. Clin Dev Immunol. 2013. PMID: 24151519 Free PMC article. - Renal-infiltrating CD11c+ cells are pathogenic in murine lupus nephritis through promoting CD4+ T cell responses.
Liao X, Ren J, Reihl A, Pirapakaran T, Sreekumar B, Cecere TE, Reilly CM, Luo XM. Liao X, et al. Clin Exp Immunol. 2017 Nov;190(2):187-200. doi: 10.1111/cei.13017. Epub 2017 Aug 18. Clin Exp Immunol. 2017. PMID: 28722110 Free PMC article.
References
- Davidson A, Aranow C. Pathogenesis and treatment of systemic lupus erythematosus nephritis. Curr Opin Rheumatol. 2006;18:468 – 475. - PubMed
- Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, Madaio MP, Davidson A. Short-term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171:489 – 497. - PubMed
- Chan O, Madaio MP, Shlomchik MJ. The roles of B cells in MRL/lpr murine lupus. Ann NY Acad Sci. 1997;815:75– 87. - PubMed
- Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407– 413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources