MEL-18 interacts with HSF2 and the SUMO E2 UBC9 to inhibit HSF2 sumoylation - PubMed (original) (raw)
MEL-18 interacts with HSF2 and the SUMO E2 UBC9 to inhibit HSF2 sumoylation
Jie Zhang et al. J Biol Chem. 2008.
Abstract
In a previous study we found that sumoylation of the DNA-binding protein heat shock factor 2 (HSF2) is up-regulated during mitosis, but the mechanism that mediates this regulation was unknown. Here we show that HSF2 interacts with the polycomb protein MEL-18, that this interaction decreases during mitosis, and that overexpression and RNA interference-mediated reduction of MEL-18 result in decreased and increased HSF2 sumoylation, respectively. Other results suggest that MEL-18 may also function to inhibit the sumoylation of other cellular proteins. The results also show that MEL-18 is able to interact with the small ubiquitin-like modifier (SUMO) ubiquitin carrier protein (E2) enzyme UBC9 and that MEL-18 inhibits the ability of UBC9 to transfer the SUMO protein to target proteins. Together, the results in this work suggest a mechanism in which MEL-18 bound to HSF2 inhibits its sumoylation by binding to and inhibiting the activity of UBC9 enzymes in the vicinity of HSF2. These results provide an explanation for how mitotic HSF2 sumoylation is regulated and suggest that MEL-18, in contrast to the sumoylation-stimulating activities of the polycomb protein PC2, actually functions like an anti-SUMO ubiquitin-protein isopeptide ligase (E3), interacting both with HSF2 and the SUMO E2 UBC9 but acting to inhibit UBC9 activity to decrease sumoylation of a target protein, in this case that of HSF2.
Figures
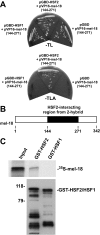
FIGURE 1.
HSF2 interacts with MEL-18. A, yeast strain pJ694A transformed with pGBD-HSF1, pGBD-HSF2, or pGBD along with pVP16-MEL-18 (amino acids 144–271) was streaked on plates lacking tryptophan and leucine (–TL) or lacking tryptophan, leucine, and alanine (–TLA). B, the schematic depicts the location within MEL-18 of the segment (amino acids 144–271) identified as an HSF2-interacting region in the yeast two-hybrid assay. C,35S-labeled in vitro translated MEL-18 was incubated with GST-HSF2 or GST-HSF1 that was bound to glutathione-agarose beads. After washing, the amount of bound 35S-labeled MEL-18 was determined by SDS-PAGE and autoradiography (upper panel). The amounts of GST-HSF2 and GST-HSF1 bound to the beads were determined by performing anti-GST Western blotting (lower panel).
FIGURE 2.
Interaction between HSF2 and MEL-18 is decreased during mitosis. Extracts of asynchronous (A) or mitotic (M) HeLa cells were immunoprecipitated using anti-HSF2 antibodies or nonspecific IgG, and the immunoprecipitates were subjected to Western blotting using anti-MEL-18 antibodies. The amounts of HSF2 in the input and anti-HSF2 immunoprecipitate samples were measured by subjecting these samples to Western blotting using goat polyclonal anti-HSF2 antibodies.
FIGURE 3.
Purified MEL-18 inhibits in vitro sumoylation of HSF2. 35S-Labeled in vitro translated HSF2 (first lane) was subjected to in vitro sumoylation in the absence of any additional purified proteins (second lane) or in the presence of purified GST-MEL-18 (third lane) or GST (fourth lane). Samples were then analyzed by SDS-PAGE and autoradiography (upper panel). The anti-GST Western blot (lower panel) shows the relative amounts of GST-MEL-18 or GST that were added to the reactions in the_third_ and fourth lanes of the upper panel, respectively. E1, ubiquitin-activating enzyme.
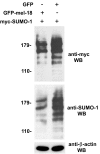
FIGURE 4.
MEL-18 inhibits sumoylation of HSF2 in vivo. A, HEK 293 cells were transfected with GFP-MEL-18 or GFP expression constructs along with Myc-SUMO-1 expression plasmid, and then extracts of the cells were subjected to immunoprecipitation (IP) with anti-HSF2 antibodies (rabbit polyclonal) or nonspecific IgG (negative control), followed by anti-Myc Western blotting (WB) to detect the sumoylated forms of HSF2. Extracts of the cells were also subjected to Western blotting using goat polyclonal HSF2 antibodies. The cell lysates were subjected to anti-GFP Western blotting to analyze expression levels of GFP-MEL-18 and GFP and to anti-β-actin Western blotting as a loading control. B, an experiment similar to that described in A was performed, except that here the cells were subjected to a 42 °C heat treatment for 60 min prior to harvesting them for immunoprecipitation analysis (to allow stress-induced HSF1 sumoylation) and that anti-HSF1 antibodies were used for the immunoprecipitation step, so that sumoylated forms of HSF1 would be detected by the subsequent anti-Myc Western blotting (detecting Myc-SUMO-1).
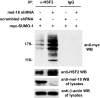
FIGURE 5.
Knockdown of MEL-18 protein levels is associated with increased HSF2 sumoylation. HeLa cells were transfected with MEL-18 shRNA or scrambled shRNA along with Myc-SUMO-1 expression plasmid, and then extracts of the cells were subjected to immunoprecipitation (IP) with anti-HSF2 antibodies (rabbit polyclonal) or nonspecific IgG (negative control), followed by anti-Myc Western blotting (WB) to detect the sumoylated forms of HSF2. Extracts of the cells were also subjected to Western blotting using goat polyclonal HSF2 antibodies. The cell lysates were subjected to anti-MEL-18 Western blotting to confirm reduction of MEL-18 protein levels by MEL-18 shRNA treatment and to anti-β-actin Western blotting as a loading control.
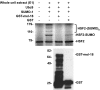
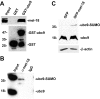
FIGURE 6.
MEL-18 interacts with the SUMO E2 enzyme UBC9. A, GST or GST-UBC9 proteins bound to glutathione-agarose beads were incubated with extracts of HeLa cells, and then after washing the amount of bound MEL-18 was determined by Western blotting using anti-MEL-18 antibodies (upper panel). The anti-GST Western blot (lower panel) shows the relative amounts of GST-UBC9 or GST that were used in the binding reactions, respectively. B, extracts of HeLa cells were subjected to immunoprecipitation using anti-MEL-18 antibodies, followed by Western blotting of the immunoprecipitates using UBC9 antibodies. C, HEK 293 cells were transfected with the GFP or GFP-MEL-18 expression constructs, and then extracts of the transfected cells were subjected to Western blot assay using anti-UBC9 antibodies to detect the non-SUMO-conjugated (18-kDa) and SUMO-containing (39-kDa) forms of UBC9. The cell lysates were also subjected to anti-β-actin Western blotting as a loading control.
FIGURE 7.
MEL-18 inhibits general protein sumoylation in cells. Extracts of HEK 293 cells transfected with GFP-MEL-18 or GFP expression constructs, along with Myc-SUMO-1 expression plasmid from the experiment shown in Fig. 4, were subjected to Western blotting (WB) using anti-Myc and anti-SUMO-1 antibodies to detect sumoylated forms of cellular proteins and to anti-β-actin Western blotting as a loading control.
Similar articles
- Mel-18 interacts with RanGAP1 and inhibits its sumoylation.
Zhang J, Sarge KD. Zhang J, et al. Biochem Biophys Res Commun. 2008 Oct 17;375(2):252-5. doi: 10.1016/j.bbrc.2008.08.012. Epub 2008 Aug 14. Biochem Biophys Res Commun. 2008. PMID: 18706886 Free PMC article. - Inhibition of DNA binding by differential sumoylation of heat shock factors.
Anckar J, Hietakangas V, Denessiouk K, Thiele DJ, Johnson MS, Sistonen L. Anckar J, et al. Mol Cell Biol. 2006 Feb;26(3):955-64. doi: 10.1128/MCB.26.3.955-964.2006. Mol Cell Biol. 2006. PMID: 16428449 Free PMC article. - Small ubiquitin-like modifier (SUMO) modification of zinc finger protein 131 potentiates its negative effect on estrogen signaling.
Oh Y, Chung KC. Oh Y, et al. J Biol Chem. 2012 May 18;287(21):17517-17529. doi: 10.1074/jbc.M111.336354. Epub 2012 Mar 30. J Biol Chem. 2012. PMID: 22467880 Free PMC article. - Cystic fibrosis transmembrane conductance regulator degradation: cross-talk between the ubiquitylation and SUMOylation pathways.
Ahner A, Gong X, Frizzell RA. Ahner A, et al. FEBS J. 2013 Sep;280(18):4430-8. doi: 10.1111/febs.12415. Epub 2013 Jul 22. FEBS J. 2013. PMID: 23809253 Free PMC article. Review. - SUMO Ubc9 enzyme as a viral target.
Varadaraj A, Mattoscio D, Chiocca S. Varadaraj A, et al. IUBMB Life. 2014 Jan;66(1):27-33. doi: 10.1002/iub.1240. Epub 2014 Jan 6. IUBMB Life. 2014. PMID: 24395713 Review.
Cited by
- Mel-18 interacts with RanGAP1 and inhibits its sumoylation.
Zhang J, Sarge KD. Zhang J, et al. Biochem Biophys Res Commun. 2008 Oct 17;375(2):252-5. doi: 10.1016/j.bbrc.2008.08.012. Epub 2008 Aug 14. Biochem Biophys Res Commun. 2008. PMID: 18706886 Free PMC article. - Small ubiquitin-like modifier (SUMO) conjugation impedes transcriptional silencing by the polycomb group repressor Sex Comb on Midleg.
Smith M, Mallin DR, Simon JA, Courey AJ. Smith M, et al. J Biol Chem. 2011 Apr 1;286(13):11391-400. doi: 10.1074/jbc.M110.214569. Epub 2011 Jan 28. J Biol Chem. 2011. PMID: 21278366 Free PMC article. - Polycomb group complexes--many combinations, many functions.
Kerppola TK. Kerppola TK. Trends Cell Biol. 2009 Dec;19(12):692-704. doi: 10.1016/j.tcb.2009.10.001. Epub 2009 Nov 4. Trends Cell Biol. 2009. PMID: 19889541 Free PMC article. Review. - The interrelationship of proteasome impairment and oligomeric intermediates in neurodegeneration.
Deger JM, Gerson JE, Kayed R. Deger JM, et al. Aging Cell. 2015 Oct;14(5):715-24. doi: 10.1111/acel.12359. Epub 2015 Jun 5. Aging Cell. 2015. PMID: 26053162 Free PMC article. Review. - PDPK1 regulates autophagosome biogenesis by binding to PIK3C3.
Hu B, Zhang Y, Deng T, Gu J, Liu J, Yang H, Xu Y, Yan Y, Yang F, Zhang H, Jin Y, Zhou J. Hu B, et al. Autophagy. 2021 Sep;17(9):2166-2183. doi: 10.1080/15548627.2020.1817279. Epub 2020 Sep 10. Autophagy. 2021. PMID: 32876514 Free PMC article.
References
- Dohmen, R. J. (2004) Biochim. Biophys. Acta 1695 113–131 - PubMed
- Johnson, E. S. (2004) Annu. Rev. Biochem. 73 355–382 - PubMed
- Hay, R. T. (2005) Mol. Cell 18 1–12 - PubMed
- Gill, G. (2005) Curr. Opin. Genet. Dev. 15 536–541 - PubMed
- Kerscher, O., Felberbaum, R., and Hochstrasser, M. (2006) Annu. Rev. Cell Dev. Biol. 22 159–180 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous