Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice - PubMed (original) (raw)
Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice
Miina K Ohman et al. Circulation. 2008.
Abstract
Background: Fat inflammation may play an important role in comorbidities associated with obesity such as atherosclerosis.
Methods and results: To first establish feasibility of fat transplantation, epididymal fat pads were harvested from wild-type C57BL/6J mice and transplanted into leptin-deficient (Lep(ob/ob)) mice. Fat transplantation produced physiological leptin levels and prevented obesity and infertility in Lep(ob/ob) mice. However, the transplanted fat depots were associated with chronically increased macrophage infiltration with characteristics identical to those observed in fat harvested from obese animals. The inflammation in transplanted adipose depots was regulated by the same factors that have been implicated in endogenous fat inflammation such as monocyte chemoattractant protein-1. To determine whether this inflamed adipose depot could affect vascular disease in mice, epididymal fat depots were transplanted into atherosclerosis-prone apolipoprotein E-deficient ApoE(-/-) mice. Plasma from ApoE(-/-) mice receiving fat transplants contained increased leptin, resistin, and monocyte chemoattractant protein-1 compared with plasma from sham-operated ApoE(-/-) mice. Furthermore, mice transplanted with visceral fat developed significantly more atherosclerosis compared with sham-operated animals, whereas transplants with subcutaneous fat did not affect atherosclerosis despite a similar degree of fat inflammation. Treatment of transplanted ApoE(-/-) mice with pioglitazone decreased macrophage content of the transplanted visceral fat pad and reduced plasma monocyte chemoattractant protein-1. Importantly, pioglitazone also reduced atherosclerosis triggered by inflammatory visceral fat but had no protective effect on atherosclerosis in the absence of the visceral fat transplantation.
Conclusions: Our results indicate that visceral adipose-related inflammation accelerates atherosclerosis in mice. Drugs such as thiazolidinediones might be a useful strategy to specifically attenuate the vascular disease induced by visceral inflammatory fat.
Figures
Figure 1
Transplanted adipose tissue displays signs of inflammation. A, Macrophages surrounding adipocyte (arrow). B, Macrophages have formed multinucleated giant cells (arrow). Staining with Mac-3; magnification ×40; scale bar=20 µm.
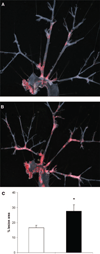
Figure 2
Effect of inflammatory fat on atherosclerosis in _ApoE_−/− mice. Representative en face view of aortic tree stained with Oil Red O of sham-operated (A) and fat-transplanted (B) _ApoE_−/− mouse. C, Fat-transplanted _ApoE_−/− mice (n=6, black bar) had significantly more atherosclerotic lesions than sham-operated (n=6, white bar) mice (_P_=0.03). All the mice were 46 to 48 weeks old at euthanasia.
Figure 3
Effect of pioglitazone on MCP-1, fat inflammation, atherosclerosis, and IL-10 in transplanted and control mice. A, Plasma MCP-1 levels in control (n=4, white bar) and pioglitazone-treated (n=3, black bar) mice 10 weeks postoperatively (_P_=0.03). All values were normalized to the average of pioglitazone-treated mice. B, IL-10 levels in transplanted fat of control (n=4, white bar) and pioglitazone-treated (n=3, black bar) _ApoE_−/− mice (_P_=0.003). C, Macrophage content of transplanted fat in control (n=4, white bar) and pioglitazone-treated (n=3, black bar) _ApoE_−/− mice (_P_=0.03). D, Atherosclerotic lesion area in control (n=4, white bar) and pioglitazone-treated (n=3, black bar) _ApoE_−/− mice (_P_=0.02). All the mice were 20 weeks old at euthanasia.
Figure 4
Subcutaneous adipose tissue transplants displayed signs of chronic inflammation such as crown-like structures (arrow) similar to those of visceral transplants. Staining with Mac-3; magnification ×40; scale bar=20 µm.
Similar articles
- Macrophage-derived apolipoprotein E ameliorates dyslipidemia and atherosclerosis in obese apolipoprotein E-deficient mice.
Atkinson RD, Coenen KR, Plummer MR, Gruen ML, Hasty AH. Atkinson RD, et al. Am J Physiol Endocrinol Metab. 2008 Feb;294(2):E284-90. doi: 10.1152/ajpendo.00601.2007. Epub 2007 Nov 20. Am J Physiol Endocrinol Metab. 2008. PMID: 18029445 - Monocyte chemoattractant protein-1 deficiency protects against visceral fat-induced atherosclerosis.
Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Russo HM, Eitzman DT. Ohman MK, et al. Arterioscler Thromb Vasc Biol. 2010 Jun;30(6):1151-8. doi: 10.1161/ATVBAHA.110.205914. Epub 2010 Mar 18. Arterioscler Thromb Vasc Biol. 2010. PMID: 20299683 Free PMC article. - Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice.
Öhman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, Eitzman DT. Öhman MK, et al. Atherosclerosis. 2011 Nov;219(1):33-9. doi: 10.1016/j.atherosclerosis.2011.07.012. Epub 2011 Jul 20. Atherosclerosis. 2011. PMID: 21835408 Free PMC article. - Visceral adipose tissue and atherosclerosis.
Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Eitzman DT. Ohman MK, et al. Curr Vasc Pharmacol. 2009 Apr;7(2):169-79. doi: 10.2174/157016109787455680. Curr Vasc Pharmacol. 2009. PMID: 19356000 Review. - Persistent low-grade inflammation and regular exercise.
Astrom MB, Feigh M, Pedersen BK. Astrom MB, et al. Front Biosci (Schol Ed). 2010 Jan 1;2(1):96-105. doi: 10.2741/s48. Front Biosci (Schol Ed). 2010. PMID: 20036931 Review.
Cited by
- Oxytocin attenuates atherosclerosis and adipose tissue inflammation in socially isolated ApoE-/- mice.
Nation DA, Szeto A, Mendez AJ, Brooks LG, Zaias J, Herderick EE, Gonzales J, Noller CM, Schneiderman N, McCabe PM. Nation DA, et al. Psychosom Med. 2010 May;72(4):376-82. doi: 10.1097/PSY.0b013e3181d74c48. Epub 2010 Apr 5. Psychosom Med. 2010. PMID: 20368478 Free PMC article. - Deletion of LDLRAP1 Induces Atherosclerotic Plaque Formation, Insulin Resistance, and Dysregulated Insulin Response in Adipose Tissue.
Leigh T, Kawai T, Preston K, Kelemen S, Okune R, St Paul A, Corbett C, Peluzzo AM, Yu J, Scalia RG, Autieri MV. Leigh T, et al. Am J Pathol. 2022 Jul;192(7):1092-1108. doi: 10.1016/j.ajpath.2022.03.014. Epub 2022 Apr 20. Am J Pathol. 2022. PMID: 35460615 Free PMC article. - Atherosclerosis and leukocyte-endothelial adhesive interactions are increased following acute myocardial infarction in apolipoprotein E deficient mice.
Wright AP, Öhman MK, Hayasaki T, Luo W, Russo HM, Guo C, Eitzman DT. Wright AP, et al. Atherosclerosis. 2010 Oct;212(2):414-7. doi: 10.1016/j.atherosclerosis.2010.06.022. Epub 2010 Jun 18. Atherosclerosis. 2010. PMID: 20619838 Free PMC article. - Lipids versus glucose in inflammation and the pathogenesis of macrovascular disease in diabetes.
Averill MM, Bornfeldt KE. Averill MM, et al. Curr Diab Rep. 2009 Feb;9(1):18-25. doi: 10.1007/s11892-009-0005-x. Curr Diab Rep. 2009. PMID: 19192420 Free PMC article. Review. - Role of visceral adipose tissue in aging.
Huffman DM, Barzilai N. Huffman DM, et al. Biochim Biophys Acta. 2009 Oct;1790(10):1117-23. doi: 10.1016/j.bbagen.2009.01.008. Epub 2009 Jan 31. Biochim Biophys Acta. 2009. PMID: 19364483 Free PMC article. Review.
References
- Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. - PubMed
- Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006;95:136–147. - PubMed
- See R, Abdullah SM, McGuire DK, Khera A, Patel MJ, Lindsey JB, Grundy SM, de Lemos JA. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol. 2007;50:752–759. - PubMed
- Lakka HM, Lakka TA, Tuomilehto J, Salonen JT. Abdominal obesity is associated with increased risk of acute coronary events in men. Eur Heart J. 2002;23:706–713. - PubMed
- Blaschke F, Spanheimer R, Khan M, Law RE. Vascular effects of TZDs: new implications. Vasc Pharmacol. 2006;45:3–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007853/HL/NHLBI NIH HHS/United States
- T32HL007853/HL/NHLBI NIH HHS/United States
- R01 HL073150/HL/NHLBI NIH HHS/United States
- I01 BX002776/BX/BLRD VA/United States
- P01 HL057346/HL/NHLBI NIH HHS/United States
- HL57346/HL/NHLBI NIH HHS/United States
- HL073150/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous