Early motor neuron pool identity and muscle nerve trajectory defined by postmitotic restrictions in Nkx6.1 activity - PubMed (original) (raw)
Early motor neuron pool identity and muscle nerve trajectory defined by postmitotic restrictions in Nkx6.1 activity
Natalia V De Marco Garcia et al. Neuron. 2008.
Abstract
The fidelity with which spinal motor neurons innervate their limb target muscles helps to coordinate motor behavior, but the mechanisms that determine precise patterns of nerve-muscle connectivity remain obscure. We show that Nkx6 proteins, a set of Hox-regulated homeodomain transcription factors, are expressed by motor pools soon after motor neurons leave the cell cycle, before the formation of muscle nerve side branches in the limb. Using mouse genetics, we show that the status of Nkx6.1 expression in certain motor neuron pools regulates muscle nerve formation, and the pattern of innervation of individual muscles. Our findings provide genetic evidence that neurons within motor pools possess an early transcriptional identity that controls target muscle specificity.
Figures
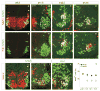
Figure 1. Expression of Nkx6 proteins in the developing spinal cord
(A–D) Patterns of expression of Nkx6.1 and Isl1(2) in lumbar spinal cord of embryonic mice. Panel A corresponds to a transverse section of the ventral spinal cord. Panels B–D correspond to transverse sections of the right ventral quadrant of the spinal cord. (E–H) Er81 expression in lumbar motor neurons. Panels C and G show the same, triple-labeled, section. (I and J) Co-expression of Nkx6.2 and Isl1(2) in a subset of motor neurons in lumbar spinal cord at e9.5 (I) and e11.5 (J). (K) Nkx6.2 expression is restricted to a subset of Lhx1on lateral LMC neurons at e12.5. (L) Percentage of LMC neurons that express Nkx6 proteins (see Experimental Procedures). Mean values (± SEM) obtained from 10 wild type embryos at each stage. m, medial; l, lateral
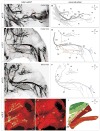
Figure 2. Genetic tracing of motor axon trajectories in the mouse hindlimb
(A, C and E) Motor axon projections in whole-mount hindlimb preparations revealed by eGFP immunoreactivity in Hb9::eGFP transgenic embryos. Confocal images were converted to black and white, and inverted to depict motor axons in black. (B, D and F) Reconstruction of main nerve trunks and muscle nerve branches. The Obturator (Obt) and Deep peroneal (Dp) nerve trunks are indicated in black and blue respectively. Individual muscle nerve branches that derive from the Obturator and Deep peroneal nerve trunks are shown in color (panels D and F). (G and H) Individual muscles delineated by expression of fast skeletal myosin (red); motor axons defined by eGFP immunoreactivity (green). The trajectory of muscle nerves is highly reproducible among different embryos (see Figure S5). (I) Diagram of nerve trunks, muscle nerve branches and muscle target fields. A, Anterior; Ab, Adductor brevis; Al, Adductor longus; Am, Adductor magnus; Bf, Biceps femoris; Di, distal; Dl, dorsal; Dp, Deep peroneal; F, Femoral; Ga, Gracilis anterior; Gp, Gracilis posterior; Gl, Gluteus; Ig, Inferior gluteal; Obt, Obturator; Oe, Obturator externus; P, Pectineus; Po, Posterior; Pr, Proximal; Qf, Quadriceps femoris; Rf, Rectus femoris; St; Semitendinosus; Sp, Superficial peroneal; T, Tibial; Ta, Tibialis anterior; Tfl, Tensor Fasciae Latae; V, Vasti; Vl, ventral. Scale bar in panels A– I: 100 μm.
Figure 3. Transcription factor expression by neurons in motor pools
(A–C) HRP-labeled motor neurons after tracer injection into the Adductor muscles at e13.5. The presence of Nkx6.1on motor neurons that are not labeled by HRP (B) is likely to reflect incomplete filling and/or retrograde transport of the tracer. 98% of HRP labeled motor neurons express Nkx6.1, and 81% of these neurons co-express Er81 (B and C) (see Supplementary Table 1). Neurons that lack Er81 expression may correspond to the Adductor brevis motor pool (see Supplementary Figure 3). Laterally positioned Nkx6.1off: Er81on neurons that are not labeled by HRP correspond to Vasti motor neurons (C). (D–F) > 99% of HRP-labeled motor neurons after tracer injection into the Adductor longus muscle (D) at e14.5 co-express Nkx6.1 (E) and Er81 (F) (see Supplementary Table 1). (G–I) > 99% of HRP-labeled motor neurons after tracer injection into the Vasti muscle at e14.5 lack Nkx6.1 expression (H). 97% of HRP-labeled motor neurons express Er81 (I, see Supplementary Table 1). (J–L) 86% of HRP-labeled motor neurons after tracer injection into the Gracilis posterior muscle (J) at e14.5 co-express Nkx6.1 (K) and Er81 (L, see Supplementary Tables 1 and 2). (M–O) 87% of HRP-labeled motor neurons after tracer injection into the Gracilis anterior muscle (M) at e14.5 lack Nkx6.1 (N) and Er81 expression (O, see Supplementary Tables 1 and 2). (P–R) > 99% of HRP-labeled motor neurons after tracer injection into the Pectineus muscle (P) at e14.5 lack Nkx6.1 (Q) and Er81 (R) expression. (S) Transcription factor expression by anatomically defined motor pools at the L2 level. The small size and inaccessibility of the Obturator externus (Oe) and the Ab muscles prevented us from labeling corresponding motor neurons, and so the position of these pools in the diagram is approximate. Rectus femoris motor neurons express Pea3 at e13.5, but not at later stages (Supplementary Table 1; data not shown). (T) Schematic representation of the trajectory of the Obturator (Obt) nerve trunk, and its side branches innervating the adductor (Al, Am and Ab) and Gracilis (Ga and Gp) muscles.
Figure 4. otor neuron columnar, divisional and pool specification in _Nkx6.1_−/− mice
(A and B) Segregation of LMCl, defined by Hb9 expression; and LMCm neurons, defined by Isl1(2) expression, occurs normally in _Nkx6.1_−/− embryos at e13.5 (B). (C) The number of somatic motor neurons at L1-L3 levels (see Experimental Procedures). Mean values (± SEM) obtained from 10 embryos of each genotype. (D) Proportion of motor neurons in columnar and divisional subtypes at L1-L3 at e13.5 and e16.5 (see Experimental Procedures). A similar proportion of motor neurons are allocated to the MMCm in _Nkx6.1_−/− and Nkx6.1+/− embryos (e13.5, n=10, p>0.05; e16.5, n=6, p>0.05). In addition, the proportion of LMCm and LMCl neurons is similar in _Nkx6.1_−/− and Nkx6.1+/− embryos (e13.5, n=10, LMCl: Nkx6.1+/− vs. _Nkx6.1_−/−, p>0.05, LMCm: Nkx6.1+/− vs. _Nkx6.1_−/−, p>0.05; e16.5, n=6, LMCl: Nkx6.1+/− vs. _Nkx6.1_−/−, p>0.05, LMCm: Nkx6.1+/− vs. _Nkx6.1_−/−; p>0.05, Student’s t test). (E and F) Adductor and Gp motor neurons maintain Er81 expression and segregate normally from Er81on: Isl1off V motor neurons in e13.5 _Nkx6.1_−/− embryos (F). However, Er81on: Isl1on, Adductor and Gp motor neurons occupy an abnormal dorso-lateral position in _Nkx6.1_−/− embryos (F). (G and H) Segregation of Adductor and V motor neurons from the Rf motor pool, delineated by Nkx6.2 expression, occurs normally in e13.5 _Nkx6.1_−/− embryos (H).
Figure 5. Defects in Adductor and Gracilis muscle nerve branches in _Nkx6.1_−/− mice
Muscle nerves were visualized by eGFP immunoreactivity on whole-mount preparations of the hindlimb. (A and B) Trajectory of the Obturator nerve in Nkx6.1+/− and _Nkx6.1_−/− embryos. (C–F) Individual Adductor and Ga muscle nerve branches are observed in Nkx6.1+/− (C and E) and _Nkx6.1_−/− (D and F) embryos. (G–J) In _Nkx6.1_−/− embryos, the Gp muscle nerve is absent and the extent of arborization of the Am, Al and Ab side branches is reduced (H and J). In Nkx6.1+/− and wild type embryos, we observed that the Ga muscle is innervated by two intramuscular nerve branches in ~50 % of cases. In contrast, in _Nkx6.1_−/− embryos, innervation of the Ga muscle by two intramuscular nerve branches in observed in only ~10% of cases. (K and L) Schematic drawings of the Obturator motor nerve and its muscle nerve branches at e16.5. Scale bar in A–L, 100μm.
Figure 6. Persistent defects in muscle innervation in _Nkx6.1_−/− embryos
Comparison of muscle nerve trajectories from Nkx6.1on and Nkx6.1off motor pools in Nkx6.1+/− and _Nkx6.1_−/− embryos, at e16.5. (A–H) Muscle nerves and motor axon fascicles in targets normally innervated by Nkx6.1on motor neurons. (I–L) Total length of motor axon fascicles within individual muscle targets. Values represent the mean (± SEM) of 3 (I–K) or 4 (L) embryos of each genotype. Motor axon fascicle length within Am, Ab, Al, Gp and Ta muscles in _Nkx6.1_−/− embryos is significantly reduced, compared to Nkx6.1+/− embryos (see Supplementary Table 4). (M–T) Muscle nerves and motor axon fascicles in muscle targets normally innervated by Nkx6.1off motor neurons. (U–X) Total length of motor axon fascicles within individual muscles. Values represent the mean (± SEM) of 3 (U–W) or 4 (X) embryos of each genotype. Motor axon fascicle length for the Ga and Vastus medialis (Vm) muscle as well as for the P and Gl muscles are similar in Nkx6.1+/− and _Nkx6.1_−/− embryos (see Supplementary Table 4). Scale bar in panels A – H and M – T, 100 μm.
Figure 7. Adductor and Gracilis motor neurons switch their muscle target in _Nkx6.1_−/−mice
(A and B) Schematic representation of the transcriptional profile of motor neurons labeled after HRP-injection into the Ga muscle in Nkx6.1+/− (A) and _Nkx6.1_−/− (B) embryos. (C, D and D′) Adductor and Gp motor neurons, delineated by Er81 expression, are labeled after injections into the Ga muscle at e14.5 in _Nkx6.1_−/− (D and D′) embryos. Er81off Ga motor neurons that project normally are also labeled after Ga injections in _Nkx6.1_−/− embryos (arrow in D′). Some of these neurons may derive from the Ab pool. (E and F) Expression of Er81 and Isl1(2) in Nkx6.1+/− (E) and _Nkx6.1_−/− embryos (F) at e14.5. The position of the Adductor and Gp pools, marked by Er81 and Isl1 co-expression, is shifted dorso-laterally in _Nkx6.1_−/− embryos (F). (G) The percentage of LMC neurons that co-express Isl1 and Er81 is similar in e14.5 _Nkx6.1_−/− (23 ± 5%, n=6) and Nkx6.1+/− (24 ± 5%; n=7, p>0.05; Student’s t test) embryos (see Experimental Procedures). (H) Quantification of HRP-labeled LMC neurons that co-express Er81 and Isl1, as a percentage of the total number of HRP-labeled motor neurons at e14.5. Values (± SEM) represent the mean of 6 _Nkx6.1_−/− and 7 Nkx6.1+/− embryos. The percentage of HRP-labeled Er81on motor neurons is higher in _Nkx6.1_−/− (56 ± 8%) than in Nkx6.1+/− embryos (12 ± 4%; p<0.001; Student’s t test). The detection of HRP-labeled, Er81on: Isl1on motor neurons in Nkx6.1+/− embryos is likely to reflect HRP leakage into the Gp muscle (see Supplementary Table 2).
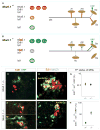
Figure 8. Rescue of muscle innervation defects by selective Nkx6.1 expression in post-mitotic motor neurons
(A and B) Expression of Nkx6.1 in progenitor cells (P) and LMC neurons in wild type embryos at e14.5 (A). Ectopic Nkx6.1 expression is detected in MMC and LMC neurons but not in interneurons (Int) (B) or progenitor cells (Figure S10) in Hb9::Nkx6.1B embryos. (C and D) Nkx6.1 expression in the LMCm, delineated by Isl1 expression, in wild type (C) and Hb9::Nkx6.1B (D) embryos at e14.5. Note the ectopic expression of Nkx6.1 in LMCm and LMCl (Lhx1on, data not shown) neurons in Hb9::Nkx6.1B embryos. (E and F) Er81 expression is detected in the Adductor, Gp and V pools in Hb9::Nkx6.1B embryos (F). (G) Percentage of LMC neurons that express Nkx6.1 at e14.5. Values (± SEM) represent the mean of 5 wild type and 5 Hb9::Nkx6.1B embryos. The proportion of Nkx6.1on motor neurons is significantly higher in Hb9::Nkx6.1B (68 ± 8%) than in wild type (38 ± 2%) (p<0.001, Student’s t test) embryos. (H) Quantification of the number of Isl1on: Er81off neurons that express Nkx6.1 at L2. There is a ~4-fold increase in the percentage of Isl1on: Er81off motor neurons that express Nkx6.1 in e14.5 Hb9::Nkx6.1B embryos (44 ± 6%, n=5) compared to that in wild type embryos (11 ± 4%, n=5; p<0.01; Student’s t test). The proportion of LMC neurons that express Er81 is similar in wild type and Hb9::Nkx6.1B embryos (data not shown). (I–N) Innervation of the Adductor and Gp muscles as well as that of the Ta muscle in Nkx6.1+/− (I and J), _Nkx6.1_−/− (K and L) and _Nkx6.1_−/− mice carrying the Hb9::Nkx6.1B transgene (M and N) at e14.5. Expression of the Hb9::Nkx6.1B transgene did not rescue the deficit in motor neuron number observed in _Nkx6.1_−/− embryos (_Nkx6.1_−/−: 1020 ± 80 motor neurons in L1/L3 segments within the LMC (unilateral): mean ± SEM, n=5 embryos; _Nkx6.1_−/−; Hb9::Nkx6.1B: 1093 ± 100, n=5; wild type: 1901 ± 60, n=5; see Experimental Procedures). Scale bar in panels I – N: 100 μm.
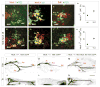
Figure 9. Ectopic expression of Nkx6.1 redirects Ga motor axons to the Gp muscle
(A and B) Pattern of Obturator muscle nerve branches in Hb9::eGFP (A) and Hb9::Nkx6.1B x Hb9::eGFP (B) mice. The Ga muscle nerve branch is greatly attenuated, whereas the Adductor and Gp muscle nerve branches appear normal in Hb9::Nkx6.1B x Hb9::eGFP embryos (B). (C and D) Few motor axons project to the Ga muscle nerve in e16.5 Hb9::Nkx6.1B x Hb9::eGFP embryos (D). The detection of a few axons in the Ga muscle in Hb9::Nkx6.1B x Hb9::eGFP mice is likely to reflect the mosaic expression of the Hb9::Nkx6.1B transgene (documented in Figure 8). (E and F) In Hb9:Nkx6.1B embryos with ≥ 60% incidence of Nkx6.1 expression (Nkx6.1on/LMC), additional Gp muscle nerve side branches are detected (red arrow in F). (G–L) Visualization of motor axons by eGFP immunoreactivity (green) and AChR by α-Bgx (red) in whole-mount muscle preparations. The Gp muscle lacks motor innervation (J) whereas motor axons innervate the Ga muscle (I) in _Nkx6.1_−/− x Hb9::eGFP embryos. Conversely, the innervation of the Ga muscle is greatly attenuated (K), whereas motor axons innervate the Gp muscle (L) in Hb9::Nkx6.1B x Hb9::eGFP embryos. (M) Transcriptional identity of motor neurons labeled after HRP-injection into the Gp muscle in Hb9::Nkx6.1B embryos. (N–O′) > 99% of HRP-labeled motor neurons express Nkx6.1 (N) after HRP injection into the Gp muscle at e14.5. A subset of HRP-labeled, Isl1on motor neurons (arrow in O) lack Er81 expression (arrow in O′) (presumptive Ga neurons) in Hb9::Nkx6.1B embryos. O and O′ show the same triple-labeled section. (P) Percentage of HRP-labeled motor neurons that lack Er81 expression after HRP injection into the Gp muscle in wild type and Hb9::Nkx6.1B embryos at L2. Values (± SEM) represent the mean of 5 embryos of each genotype. The percentage of HRP-labeled Nkx6.1on: Er81off motor neurons is higher in Hb9::Nkx6.1B (61 ± 12%) than in wild type embryos (11 ± 4%; p<0.001; Student’s t test). Scale bars in panels A - I, 100 μm. m, medial; l, lateral
Similar articles
- A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity.
Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. Dasen JS, et al. Cell. 2005 Nov 4;123(3):477-91. doi: 10.1016/j.cell.2005.09.009. Cell. 2005. PMID: 16269338 - Specification of motor axon trajectory by ephrin-B:EphB signaling: symmetrical control of axonal patterning in the developing limb.
Luria V, Krawchuk D, Jessell TM, Laufer E, Kania A. Luria V, et al. Neuron. 2008 Dec 26;60(6):1039-53. doi: 10.1016/j.neuron.2008.11.011. Neuron. 2008. PMID: 19109910 - Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1.
Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Dasen JS, et al. Cell. 2008 Jul 25;134(2):304-16. doi: 10.1016/j.cell.2008.06.019. Cell. 2008. PMID: 18662545 - Transcriptional codes and the control of neuronal identity.
Shirasaki R, Pfaff SL. Shirasaki R, et al. Annu Rev Neurosci. 2002;25:251-81. doi: 10.1146/annurev.neuro.25.112701.142916. Epub 2002 Mar 27. Annu Rev Neurosci. 2002. PMID: 12052910 Review. - Transcriptional mechanisms controlling motor neuron diversity and connectivity.
Dalla Torre di Sanguinetto SA, Dasen JS, Arber S. Dalla Torre di Sanguinetto SA, et al. Curr Opin Neurobiol. 2008 Feb;18(1):36-43. doi: 10.1016/j.conb.2008.04.002. Epub 2008 Jun 2. Curr Opin Neurobiol. 2008. PMID: 18524570 Review.
Cited by
- Genetic and functional modularity of Hox activities in the specification of limb-innervating motor neurons.
Lacombe J, Hanley O, Jung H, Philippidou P, Surmeli G, Grinstein J, Dasen JS. Lacombe J, et al. PLoS Genet. 2013;9(1):e1003184. doi: 10.1371/journal.pgen.1003184. Epub 2013 Jan 24. PLoS Genet. 2013. PMID: 23359544 Free PMC article. - Gamma and alpha motor neurons distinguished by expression of transcription factor Err3.
Friese A, Kaltschmidt JA, Ladle DR, Sigrist M, Jessell TM, Arber S. Friese A, et al. Proc Natl Acad Sci U S A. 2009 Aug 11;106(32):13588-93. doi: 10.1073/pnas.0906809106. Epub 2009 Jul 27. Proc Natl Acad Sci U S A. 2009. PMID: 19651609 Free PMC article. - From circuits to behaviour: motor networks in vertebrates.
Garcia-Campmany L, Stam FJ, Goulding M. Garcia-Campmany L, et al. Curr Opin Neurobiol. 2010 Feb;20(1):116-25. doi: 10.1016/j.conb.2010.01.002. Epub 2010 Feb 6. Curr Opin Neurobiol. 2010. PMID: 20138753 Free PMC article. Review. - Directed differentiation of human-induced pluripotent stem cells generates active motor neurons.
Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, Clark AT, Goldman SA, Plath K, Wiedau-Pazos M, Kornblum HI, Lowry WE. Karumbayaram S, et al. Stem Cells. 2009 Apr;27(4):806-11. doi: 10.1002/stem.31. Stem Cells. 2009. PMID: 19350680 Free PMC article. - Topoisomerase IIβ Selectively Regulates Motor Neuron Identity and Peripheral Connectivity through Hox/Pbx-Dependent Transcriptional Programs.
Edmond M, Hanley O, Philippidou P. Edmond M, et al. eNeuro. 2017 Dec 14;4(6):ENEURO.0404-17.2017. doi: 10.1523/ENEURO.0404-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29379870 Free PMC article.
References
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. - PubMed
- Broihier HT, Kuzin A, Zhu Y, Odenwald W, Skeath JB. Drosophila homeodomain protein Nkx6 coordinates motoneuron subtype identity and axonogenesis. Development. 2004;131:5233–5242. - PubMed
- Carpenter EM. Hox genes and spinal cord development. Dev Neurosci. 2002;24:24–34. - PubMed
- Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. - PubMed
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases