Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3 - PubMed (original) (raw)
. 2008 Jan 24;57(2):263-75.
doi: 10.1016/j.neuron.2007.11.032.
Omar Akil, Eunyoung Yi, Christopher M Weber, Lisa Grant, Jong Yoo, Amanda Clause, Karl Kandler, Jeffrey L Noebels, Elisabeth Glowatzki, Lawrence R Lustig, Robert H Edwards
Affiliations
- PMID: 18215623
- PMCID: PMC2293283
- DOI: 10.1016/j.neuron.2007.11.032
Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3
Rebecca P Seal et al. Neuron. 2008.
Abstract
The expression of unconventional vesicular glutamate transporter VGLUT3 by neurons known to release a different classical transmitter has suggested novel roles for signaling by glutamate, but this distribution has raised questions about whether the protein actually contributes to glutamate release. We now report that mice lacking VGLUT3 are profoundly deaf due to the absence of glutamate release from hair cells at the first synapse in the auditory pathway. The early degeneration of some cochlear ganglion neurons in knockout mice also indicates an important developmental role for the glutamate released by hair cells before the onset of hearing. In addition, the mice exhibit primary, generalized epilepsy that is accompanied by remarkably little change in ongoing motor behavior. The glutamate release conferred by expression of VGLUT3 thus has an essential role in both function and development of the auditory pathway, as well as in the control of cortical excitability.
Figures
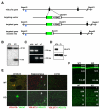
Figure 1. Targeted Disruption of the VGLUT3 Locus
(A) Restriction maps of the WT VGLUT3 mouse gene locus, the targeting construct and the properly recombined allele. In the recombined allele, most of exon 2 is replaced by EGFP (green box) and the positive selectable marker for resistance to neomycin (neo). The neo gene, flanked by loxP sites was deleted by mating mice heterozygous for VGLUT3 to β-actin-cre mice that express cre recombinase in the germline. Exon 2 encodes the first transmembrane domain and part of the first lumenal loop of VGLUT3, including glycosylation sites. The 5' probe (blue box) used to identify properly targeted ES cell clones resides upstream of the left arm. (B) Southern blot of _Bam_H1-cut genomic DNA from three neomycin resistant ES cell clones. The wild type allele results in an ∼22 kb band, whereas the targeted allele results in an ∼8 kb fragment. (C) PCR amplification of genomic tail DNA from wild type (+/+), heterozygous (+/−) and homozygous (−/−) VGLUT3 knock-out (KO) mice. A 720 bp band is amplified from the wild type allele, and a 330 bp band from the recombined allele. (D) Western blot of crude brain synaptic vesicles prepared from wild type (+/+) and VGLUT3 KO (−/−) littermates probed with antibodies to VGLUT3 and synaptophysin (syp). (E) Striatal, hippocampal and cortical slices from wild type (WT) and VGLUT3 KO mice were immunostained for VGLUT3 (red) and VAChT, VGLUT1 or VGLUT2 (all green). (F) Hippocampal region CA1 (top) and parietal cortex (bottom) of VGLUT3 KO mice show no difference from WT in overall morphology assessed by Nissl stain (green).
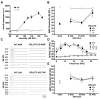
Figure 2. Sensorineural Deafness in VGLUT3 KO Mice
(A) Acoustic startle response of VGLUT3 KO mice (open circles, n=7) and WT littermates (filled circles, n= 7) at sound intensities from 80 to 125 dB sound pressure level (SPL). KO mice show no response at any intensity. (B) Auditory brainstem response (ABR) thresholds to click stimuli (up to 91.5 dB SPL) or pure tones (8, 16 and 32 kHz, at up to 88.1 dB SPL) for P21 WT (filled circles, n=5), VGLUT3 heterozygotes (gray circles, n=5) and VGLUT3 KO mice (open circles, n=5). VGLUT3 KO mice show no response. Wild type mice show an impairment at high frequency, consistent with impaired high frequency hearing in the C57Bl/6 mice to which the VGLUT3 KO were back-crossed. (C) Representative ABR (P21) and compound action potentials (CAP) (P14-17) for WT and VGLUT3 KO mice. A small stimulus artifact that precedes the onset of the CAP in WT mice is all that remains in the KO. (D) Distortion product otoacoustic emissions spectra (DPOAE) measured at 65 dB SPL show no difference between WT (filled circles, n=5), VGLUT3 heterozygotes (gray circles, n=5) and VGLUT3 KO mice (open circles, n=5). Nf is the noise floor measurement. (E) ABR thresholds to click stimuli (up to 91.5 dB SPL) or pure tones (8, 16 and 32 kHz, at up to 88.1 dB SPL) for P21 WT (filled circles, n=4) show no difference from VGLUT1 heterozygotes (gray circles, n=5) and VGLUT1 KO mice (open circles, n=4). Error bars indicate the standard error of the mean.
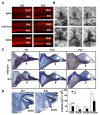
Figure 3. The Loss of VGLUT3 Affects Inner Hair Cell Ribbons and Spiral Ganglion Cell Survival
(A) Surface preparation of sensory hair cells from the apex, mid-turn and base of P21 VGLUT3 KO mice shows no difference from WT by staining with rhodamine-phalloidin. (B) Electron microscopy of inner hair cells (IHC) from P21 VGLUT3 KO (−/−) mice shows abnormally thin, elongated ribbons. IHC ribbon synapses from WT littermates (+/+) are shown for reference. 40,000x magnification. (C) Sections from the mid-turn of WT and VGLUT3 KO cochlea at P4, P10 and P21 were stained with osmium tetroxide and toluidine blue. Spiral ganglia (outlined in red) from KO animals show fewer cells than WT at P10 and P21. The nerve fiber bundle (arrows) is also thinned in VGLUT3 KO mice. (D) Volume of the posteroventral cochlear nucleus (PVCN) and AVCN but not dorsal cochlear nucleus (DCN) is significantly reduced in VGLUT3 KO mice. (n=4, p<0.01, Student's t-test)
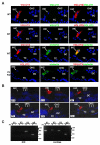
Figure 4. VGLUT Expression in the Organ of Corti
(A) Immunofluorescence for VGLUT3 (red) and either VGLUT1 (green) or VGLUT2 (green) in cochlea of P21 WT mice shows expression of only VGLUT3 by IHCs. VGLUT3 immunoreactivity (red) localizes to the IHC in a pattern resembling that of the synaptic vesicle protein otoferlin (green). P21 VGLUT3 KO mice show no immunoreactivity for VGLUT3 (red), while otoferlin expression appears normal (green). Nuclei are stained with DAPI (blue). (B) VGLUT3 (red) becomes detectable by immunofluorescence in the cochlea of WT mice by E19. OHC, outer hair cell; TC, Tunnel of Corti; DC, Deiters cells. (C) RT-PCR of P13-14 rat IHCs shows expression of only the VGLUT3 isoform and the nAChR subunit α9, a gene expressed exclusively by hair cells within the cochlea. The cochlea as a whole expresses all three VGLUT isoforms and the nAChR α9. No products were detected when RT-PCR was performed in the absence of reverse transcriptase (-RT).
Figure 5. VGLUT3 KO Mice Exhibit Developmentally Regulated Inner Hair Cell Conductances Similar to WT
(A) IHC calcium currents recorded from a P7 VGLUT3 KO mouse in response to 100 ms depolarizations from -84 mV to +16 mV in 10 mV increments. (B) Similar current-voltage relationships for calcium currents from P7-8 VGLUT3 KO (n=9) and WT IHCs (n=9). Current amplitudes were measured after full activation, and the values presented are mean ± S.E. (C) Calcium action potentials produced by current injection into a P8 VGLUT3 KO IHC. 100 ms current steps were applied from −40 pA in 10 pA increments. In this cell, calcium action potentials were generated with +20 pA current injection. Inset shows spontaneous calcium action potentials recorded from a VGLUT3 KO IHC in the absence of current injection. Scale: 20 mV and 200 ms. (D) Using the same protocol, a P16 VGLUT3 KO IHC shows no calcium action potentials. (E, F) Potassium currents recorded from P8 (E) and P16 (F) VGLUT3 KO IHCs in response to 100 ms voltage steps from –104 mV to +26 mV in 10 mV increments from a holding potential of −84 mV (nominal voltages). Actual voltages reached after correcting for series resistance error are shown next to selected traces. (G) Depolarization with 40 mM extracellular potassium activates synaptic currents from cholinergic efferents in a VGLUT3 KO IHC at P7. Inset shows single synaptic currents on an expanded timescale (30 pA, 250 ms). (H) No synaptic currents are detected in a VGLUT3 KO IHC at P16. The slow rise in inward current in both (G) and (H) results from the change in K+ equilibrium potential produced by addition of 40 mM K+.
Figure 6. Absence of synaptic activity in the afferent terminals of VGLUT3 KO mice
(A,B) I-V relationships recorded from IHC afferent terminals in response to 200 ms voltage steps from −104 to +36 mV in 10 mV increments (holding potential −84 mV) show no difference between WT (A) and VGLUT3 KO mice (B). Inset in (B) shows Na currents on an expanded time scale for voltage steps from −84 to −4 mV (0.5 nA, 1 ms). (C,D) Afferent terminal responses to depolarization with 40 mM potassium (holding potential −94 mV) show AMPA receptor-mediated synaptic currents completely blocked by 10 μM NBQX in WT animals (C). Inset shows individual synaptic currents on an expanded time scale (50 pA, 2 ms). (D) No synaptic activity was detected in the afferent terminals of VGLUT3 KO mice. The slow rise in inward current in (C) and (D) reflects the change in K+ equilibrium potential due to addition of 40 mM K+. (E,F) 100 μM kainate (KA) (with 100 μM CTZ to prevent AMPA receptor des ensitization) evokes inward currents in both WT (E) and VGLUT3 KO (F) afferent terminals (holding potential –94 mV). Kainate-evoked currents were completely blocked by 10 μM NBQX.
Figure 7. VGLUT3 KO Mice Exhibit Seizures and Frequent Interictal Spike Discharges
(A) Chronic EEG recording shows two distinct types of interictal discharge in VGLUT3 KO mice (−/−), one a long-lasting spike-wave complex, and the other a positive-going sharp spike discharge. Mice heterozygous for VGLUT3 (+/−) also show sharp interictal spike discharges, but no discharges or baseline EEG abnormalities were recorded from wild type (+/+) littermates. **(B)**VGLUT3 KO mice show prolonged generalized spike-wave seizure discharges with no apparent effects on motor behavior (Supplementary Movies S2 and S3). All leads are recorded using a digital average reference source. Calibration bars: (A) 1.25 sec, 500 μV, (B) 1 sec, 500 μV. Ele ctrode montage: LF left frontal, RF right frontal, T temporal, P parietal, O occipital.
Comment in
- Into great silence without VGLUT3.
Ahnert-Hilger G, Jahn R. Ahnert-Hilger G, et al. Neuron. 2008 Jan 24;57(2):173-4. doi: 10.1016/j.neuron.2008.01.009. Neuron. 2008. PMID: 18215615
Similar articles
- Vesicular Glutamatergic Transmission in Noise-Induced Loss and Repair of Cochlear Ribbon Synapses.
Kim KX, Payne S, Yang-Hood A, Li SZ, Davis B, Carlquist J, V-Ghaffari B, Gantz JA, Kallogjeri D, Fitzpatrick JAJ, Ohlemiller KK, Hirose K, Rutherford MA. Kim KX, et al. J Neurosci. 2019 Jun 5;39(23):4434-4447. doi: 10.1523/JNEUROSCI.2228-18.2019. Epub 2019 Mar 29. J Neurosci. 2019. PMID: 30926748 Free PMC article. - Outer Hair Cell Glutamate Signaling through Type II Spiral Ganglion Afferents Activates Neurons in the Cochlear Nucleus in Response to Nondamaging Sounds.
Weisz CJC, Williams SG, Eckard CS, Divito CB, Ferreira DW, Fantetti KN, Dettwyler SA, Cai HM, Rubio ME, Kandler K, Seal RP. Weisz CJC, et al. J Neurosci. 2021 Mar 31;41(13):2930-2943. doi: 10.1523/JNEUROSCI.0619-20.2021. Epub 2021 Feb 11. J Neurosci. 2021. PMID: 33574178 Free PMC article. - Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice.
Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, Rebillard G, Lenoir M, Eybalin M, Delprat B, Sivakumaran TA, Giros B, El Mestikawy S, Moser T, Smith RJ, Lesperance MM, Puel JL. Ruel J, et al. Am J Hum Genet. 2008 Aug;83(2):278-92. doi: 10.1016/j.ajhg.2008.07.008. Am J Hum Genet. 2008. PMID: 18674745 Free PMC article. - Review of hair cell synapse defects in sensorineural hearing impairment.
Moser T, Predoehl F, Starr A. Moser T, et al. Otol Neurotol. 2013 Aug;34(6):995-1004. doi: 10.1097/MAO.0b013e3182814d4a. Otol Neurotol. 2013. PMID: 23628789 Review. - Contribution of Vesicular Glutamate Transporters to Stress Response and Related Psychopathologies: Studies in VGluT3 Knockout Mice.
Horváth HR, Fazekas CL, Balázsfi D, Jain SK, Haller J, Zelena D. Horváth HR, et al. Cell Mol Neurobiol. 2018 Jan;38(1):37-52. doi: 10.1007/s10571-017-0528-7. Epub 2017 Aug 3. Cell Mol Neurobiol. 2018. PMID: 28776199 Free PMC article. Review.
Cited by
- Cochlear kainate receptors.
Peppi M, Landa M, Sewell WF. Peppi M, et al. J Assoc Res Otolaryngol. 2012 Apr;13(2):199-208. doi: 10.1007/s10162-011-0309-9. Epub 2012 Jan 10. J Assoc Res Otolaryngol. 2012. PMID: 22231646 Free PMC article. - Potential treatments for genetic hearing loss in humans: current conundrums.
Minoda R, Miwa T, Ise M, Takeda H. Minoda R, et al. Gene Ther. 2015 Aug;22(8):603-9. doi: 10.1038/gt.2015.27. Epub 2015 Mar 17. Gene Ther. 2015. PMID: 25781649 Review. - Effects of repeated "benign" noise exposures in young CBA mice: shedding light on age-related hearing loss.
Wang Y, Ren C. Wang Y, et al. J Assoc Res Otolaryngol. 2012 Aug;13(4):505-15. doi: 10.1007/s10162-012-0329-0. Epub 2012 Apr 25. J Assoc Res Otolaryngol. 2012. PMID: 22532192 Free PMC article. - Vesicular and plasma membrane transporters for neurotransmitters.
Blakely RD, Edwards RH. Blakely RD, et al. Cold Spring Harb Perspect Biol. 2012 Feb 1;4(2):a005595. doi: 10.1101/cshperspect.a005595. Cold Spring Harb Perspect Biol. 2012. PMID: 22199021 Free PMC article. Review. - Exposure to sodium salicylate disrupts VGLUT3 expression in cochlear inner hair cells and contributes to tinnitus.
Zhang W, Peng Z, Yu S, Song QL, Qu TF, Liu K, Gong SS. Zhang W, et al. Physiol Res. 2020 Feb 19;69(1):181-190. doi: 10.33549/physiolres.934180. Epub 2019 Dec 19. Physiol Res. 2020. PMID: 31852197 Free PMC article.
References
- Abercrombie M. Estimation of Nuclear Population from MIcrotome Sections. Anatomical Record. 1946;94:239–247. - PubMed
- Bobbin RP. Glutamate and aspartate mimic the afferent transmitter in the cochlea. Exp Brain Res. 1979;34:389–393. - PubMed
- Boulland JL, Qureshi T, Seal RP, Rafiki A, Gundersen V, Bergersen LH, Fremeau RT, Jr., Edwards RH, Storm-Mathisen J, Chaudhry FA. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480:264–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials