The tip growth apparatus of Aspergillus nidulans - PubMed (original) (raw)
The tip growth apparatus of Aspergillus nidulans
Naimeh Taheri-Talesh et al. Mol Biol Cell. 2008 Apr.
Abstract
Hyphal tip growth in fungi is important because of the economic and medical importance of fungi, and because it may be a useful model for polarized growth in other organisms. We have investigated the central questions of the roles of cytoskeletal elements and of the precise sites of exocytosis and endocytosis at the growing hyphal tip by using the model fungus Aspergillus nidulans. Time-lapse imaging of fluorescent fusion proteins reveals a remarkably dynamic, but highly structured, tip growth apparatus. Live imaging of SYNA, a synaptobrevin homologue, and SECC, an exocyst component, reveals that vesicles accumulate in the Spitzenkörper (apical body) and fuse with the plasma membrane at the extreme apex of the hypha. SYNA is recycled from the plasma membrane by endocytosis at a collar of endocytic patches, 1-2 mum behind the apex of the hypha, that moves forward as the tip grows. Exocytosis and endocytosis are thus spatially coupled. Inhibitor studies, in combination with observations of fluorescent fusion proteins, reveal that actin functions in exocytosis and endocytosis at the tip and in holding the tip growth apparatus together. Microtubules are important for delivering vesicles to the tip area and for holding the tip growth apparatus in position.
Figures
Figure 1.
GFP-actin and GFP-tropomyosin localization. (A) A maximum intensity projection of a Z-series stack from a set of time-lapse images showing actin in two swollen conidia (asexual spores). In the lower conidium, the actin has accumulated (arrow) at a site at which germ tube emergence is occurring. (B) A maximum intensity projection of a Z-series stack through a germling. The remnant of the swollen conidium is visible at the right. GFP-actin patches are clustered near the tip and scattered behind the tip. (C) A single focal plane image of a rapidly growing tip cell. Because it is a single focal plane image rather than a projection, the cortical location of the actin patches is clear. Note the single actin spot at the hyphal apex (arrow). Such spots are presumably present in germlings, but they are difficult to distinguish among the actin patches. (D and E) Localization of N-terminally tagged tropomyosin at a growing hyphal tip. Tropomyosin localizes to the Spitzenkörper (arrows) and to microfilaments (some of which are designated with arrowheads). Microfilaments pass near the plasma membrane and extend pass the region in which the collar of actin patches is found. Two such microfilaments are shown in E (arrowheads). All panels are the same magnification and were deconvolved to reduce background fluorescence.
Figure 2.
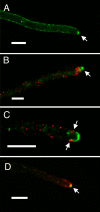
Localization of SSOA, SYNA, ABPA, and SECC in living cells. (A) Hyphal tip cell expressing GFP-SSOA. The image is from a Z-series stack (0.5-μm vertical spacing between captures) that has been deconvolved. The image is a maximum intensity projection image of three consecutive sections. SSOA is predominantly at the plasma membrane, and there is a region of greater SSOA concentration at the hyphal apex (arrow) and a region of reduced concentration immediately posterior to the apical SSOA patch. The SSOA is not evenly distributed through the plasma membrane but has a patchy or punctate distribution. (B) ABPA-mRFP (red) and GFP-SYNA (green). It is a maximum intensity projection of a deconvolved Z-series stack (0.5-μm vertical spacing between captures) through the entire hyphal tip cell. The threshold for the green channel was chosen such that the Spitzenkörper is clear (arrow), but the GFP-SYNA at the membrane anterior to the ABPA patches is less clear. The ABPA patches form a collar around the hypha behind the apex and the Spitzenkörper. (C) A single optical section of a hyphal tip cell expressing GFP-SYNA and ABPA-mRFP. The Spitzenkörper was not particularly prominent in this cell, and it was mainly located in a different optical plane. This allows the SYNA localization at the plasma membrane to be seen clearly. SYNA is at the plasma membrane between the apex and the collar of ABPA patches. In this focal plane, four ABPA patches from the collar are visible (arrows). There is a particularly prominent internal SYNA-containing structure that seems too large to be a vesicle immediately posterior to the top patches. SYNA-containing structures varied considerably in size, and we speculate that this could be a Golgi apparatus or endosome. The cortical location of the ABPA patches is evident near the tip. The hypha, however, is at a slight angle relative to the plane of optical section, and this makes some of the ABPA patches seem internal, although they are, in fact, cortical. The ABPA patches further away from the tip are actually at the surface of the cell nearest the reader. (D) mCherry-SYNA (red) and SECC-GFP (green). SECC, an exocyst component, localizes to the apex (arrow). Bars, 5 μm.
Figure 3.
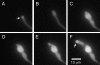
Cytochalasin A treatment and washout in a strain expressing GFP-actin. These are maximum intensity projections of Z-series stacks from a time-lapse data set (see Supplemental Video3.mov). (A) GFP-actin localization in a hyphal tip immediately before cytochalasin A addition. The collar of actin patches is visible (arrow). (B) Fifteen minutes after cytochalasin A addition and immediately before cytochalasin A washout. Actin patches have dissolved, and the tip has swollen. (C) Seventy minutes after cytochalasin A washout. The tip has swollen further. (D) Eighty minutes after cytochalasin A washout. A hyphal tip has emerged. (E) Ninety minutes after cytochalasin A washout. The hyphal tip has begun to extend. (F) One hundred minutes after cytochalasin A washout. A collar of actin patches has reformed (arrow). The new hypha is larger in diameter than the hypha before cytochalasin A treatment.
Figure 4.
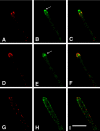
GFP-SYNA and ABPA-mRFP in a cytochalasin A-treated hypha. Each frame is a single focal plane image from deconvolved Z-series stacks taken as part of a time-lapse series. A, D, and G show ABPA-mRFP. B, E, and H show GFP-SYNA. C, F, and I are color composites. (A–C) A hyphal tip immediately before cytochalasin A addition. The Spitzenkörper is designated with an arrow in B. GFP-SYNA is in the Spitzenkörper, and vesicles are in the hypha (fainter) and at the plasma membrane between the hyphal apex and the collar of ABPA patches. (D–F) The same hyphal tip 5 min after cytochalasin A addition. The vesicular cluster of the Spitzenkörper has floated back from the hyphal apex (arrow in E), and the ABPA-mRFP patches have moved to the tip area (D). (G–I) The same hypha 1 h after cytochalasin A addition. GFP-SYNA now occupies a larger area of the plasma membrane at the tip region (H), although the fluorescence signal is weaker, indicating less GFP-SYNA per unit area. mRFP-ABPA is now apparently randomly distributed in the cortex (G). Bar (I), 10 μm.
Figure 5.
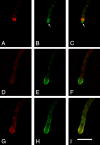
GFP-SYNA and ABPA-mRFP in a latrunculin B treated hypha. Each frame is from deconvolved Z-series stacks taken as part of a time-lapse series (see Supplemental Video4.mov). A, D, and G show ABPA-mRFP. B, E, and H show GFP-SYNA. C, F, and I are color composites. (A–C) A single focal plane image of a hyphal tip taken 10 min before latruculin B addition. The Spitzenkörper is designated with an arrow. GFP-SYNA is in the Spitzenkörper and in vesicles near the tip and further back in the hypha. In this case, there are so many vesicles in the vicinity of the tip that it is difficult to distinguish the GFP-SYNA at the membrane in the region between the apex and the ABPA patches from the vesicles. (D–F) A single focal plane image of the same hyphal tip 15 min after latrunculin B addition. The tip has bulged and the ABPA-mRFP patches have dispersed. The GFP-SYNA at the membrane is now clearly visible because there are fewer vesicles in the tip area and it occupies a larger area than before latrunculin B addition. (G–I) A projection of two consecutive Z-series images of the same hypha 60 min after latrunculin B addition. GFP-SYNA now occupies a larger area of the plasma membrane at the tip region (H), although the fluorescence signal is weaker, indicating less GFP-SYNA per unit area. Bar (I), 10 μm.
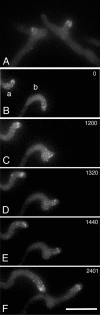
Figure 6.
Benomyl treatment and recovery in a strain expressing GFP-actin. (A) Abnormal branching after benomyl treatment. Lateral branching does not normally occur in the tip cell. (B–F) Recovery of a different pair of hyphae after benomyl washout. The time after benomyl washout (in seconds) is given at the upper right of each panel. At T = 0, the hyphal tips display obvious curvature. Over time, the actin patches become more organized and the hyphal tip displays more rapid and straighter growth. Bar (F), 10 μm.
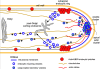
Figure 7.
A model for tip growth in A. nidulans. The Spitzenkörper is not specifically labeled but includes (but is not necessarily limited to) the vesicle cluster and the apical actin cluster as well as the apical SSOA patch, SECC, and apical SEPA, which are not shown. Secretory vesicles containing components necessary for tip growth are transported toward the tip along microtubules powered by kinesin molecules (data not shown). The + ends of microtubules are extremely dynamic. In some cases, they transiently contact the vesicle cluster. In such cases, the secretory vesicles could be transferred directly from microtubules to the cluster. In other cases, secretory vesicles presumably fall off of the microtubule as the + end disassembles, and they are transported to the vesicle cluster by myosin molecules (not shown) on actin cables. Vesicles fuse with the plasma membrane, releasing their contents and the components of the membranes of the secretory vesicles (here represented by SYNA) become incorporated into the plasma membrane. As the tip grows, the ring of actin/ABPA endocytic patches moves forward, removing SYNA and other vesicle membrane components from the plasma membrane and incorporating them into endocytic vesicles for recycling. Although we do not have direct evidence, information from other systems and from Peñalva, Rodríguez and Arenza (personal communication) indicates that these vesicles move to the post-Golgi sorting endosome by mechanisms that are not yet defined. From this compartment, SYNA-containing membranes move away from the tip on microtubules, powered by dynein, to be eventually incorporated into Golgi-derived secretory vesicles containing cell wall biosynthetic enzymes and wall precursors.
Similar articles
- Endocytic machinery protein SlaB is dispensable for polarity establishment but necessary for polarity maintenance in hyphal tip cells of Aspergillus nidulans.
Hervás-Aguilar A, Peñalva MA. Hervás-Aguilar A, et al. Eukaryot Cell. 2010 Oct;9(10):1504-18. doi: 10.1128/EC.00119-10. Epub 2010 Aug 6. Eukaryot Cell. 2010. PMID: 20693304 Free PMC article. - Rapid tip-directed movement of Golgi equivalents in growing Aspergillus nidulans hyphae suggests a mechanism for delivery of growth-related materials.
Hubbard MA, Kaminskyj SGW. Hubbard MA, et al. Microbiology (Reading). 2008 May;154(Pt 5):1544-1553. doi: 10.1099/mic.0.2007/014811-0. Microbiology (Reading). 2008. PMID: 18451063 - The role of actin, fimbrin and endocytosis in growth of hyphae in Aspergillus nidulans.
Upadhyay S, Shaw BD. Upadhyay S, et al. Mol Microbiol. 2008 May;68(3):690-705. doi: 10.1111/j.1365-2958.2008.06178.x. Epub 2008 Mar 4. Mol Microbiol. 2008. PMID: 18331474 - Fluorescent proteins illuminate the structure and function of the hyphal tip apparatus.
Sudbery P. Sudbery P. Fungal Genet Biol. 2011 Sep;48(9):849-57. doi: 10.1016/j.fgb.2011.02.004. Epub 2011 Mar 22. Fungal Genet Biol. 2011. PMID: 21362491 Review. - A role for endocytic recycling in hyphal growth.
Shaw BD, Chung DW, Wang CL, Quintanilla LA, Upadhyay S. Shaw BD, et al. Fungal Biol. 2011 Jun;115(6):541-6. doi: 10.1016/j.funbio.2011.02.010. Epub 2011 Feb 19. Fungal Biol. 2011. PMID: 21640317 Review.
Cited by
- Comparative live-cell imaging analyses of SPA-2, BUD-6 and BNI-1 in Neurospora crassa reveal novel features of the filamentous fungal polarisome.
Lichius A, Yáñez-Gutiérrez ME, Read ND, Castro-Longoria E. Lichius A, et al. PLoS One. 2012;7(1):e30372. doi: 10.1371/journal.pone.0030372. Epub 2012 Jan 24. PLoS One. 2012. PMID: 22291944 Free PMC article. - Sphingolipid biosynthetic pathway is crucial for growth, biofilm formation and membrane integrity of Scedosporium boydii.
Rollin-Pinheiro R, Rochetti VP, Xisto MIDDS, Liporagi-Lopes LC, Bastos B, Rella A, Singh A, Rozental S, Del Poeta M, Barreto-Bergter E. Rollin-Pinheiro R, et al. Future Med Chem. 2019 Nov;11(22):2905-2917. doi: 10.4155/fmc-2019-0186. Epub 2019 Nov 12. Future Med Chem. 2019. PMID: 31713454 Free PMC article. - Organization and dynamics of the Aspergillus nidulans Golgi during apical extension and mitosis.
Pantazopoulou A, Peñalva MA. Pantazopoulou A, et al. Mol Biol Cell. 2009 Oct;20(20):4335-47. doi: 10.1091/mbc.e09-03-0254. Epub 2009 Aug 19. Mol Biol Cell. 2009. PMID: 19692566 Free PMC article. - F-actin dynamics in Neurospora crassa.
Berepiki A, Lichius A, Shoji JY, Tilsner J, Read ND. Berepiki A, et al. Eukaryot Cell. 2010 Apr;9(4):547-57. doi: 10.1128/EC.00253-09. Epub 2010 Feb 5. Eukaryot Cell. 2010. PMID: 20139238 Free PMC article. - The type V myosin-containing complex HUM is a RAB11 effector powering movement of secretory vesicles.
Pinar M, Alonso A, de Los Ríos V, Bravo-Plaza I, de la Gandara Á, Galindo A, Arias-Palomo E, Peñalva MÁ. Pinar M, et al. iScience. 2022 Jun 2;25(7):104514. doi: 10.1016/j.isci.2022.104514. eCollection 2022 Jul 15. iScience. 2022. PMID: 35754728 Free PMC article.
References
- Araujo-Bazán L., Peñalva M. A., Espeso E. A. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol. Microbiol. 2008;67:891–905. - PubMed
- Bartnicki-Garcia S. Role of vesicles in apical growth and a new mathematical model of hyphal morphogenesis. In: Heath I. B., editor. Tip Growth in Plant and Fungal Cells. San Diego, CA: Academic Press; 1990. pp. 211–232.
- Bartnicki-Garcia S., Hergert F., Gierz G. Computer simulation of fungal morphogenesis and the mathematical basis for hyphal (tip) growth. Protoplasma. 1989;153:46–57.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous