Protein folding studied by single-molecule FRET - PubMed (original) (raw)
Review
Protein folding studied by single-molecule FRET
Benjamin Schuler et al. Curr Opin Struct Biol. 2008 Feb.
Abstract
A complete understanding of a protein-folding mechanism requires description of the distribution of microscopic pathways that connect the folded and unfolded states. This distribution can, in principle, be described by computer simulations and theoretical models of protein folding, but is hidden in conventional experiments on large ensembles of molecules because only average properties are measured. A long-term goal of single-molecule fluorescence studies is to time-resolve the structural events as individual molecules make transitions between folded and unfolded states. Although such studies are still in their infancy, the work till now shows great promise and has already produced novel and important information on current issues in protein folding that has been impossible or difficult to obtain from ensemble measurements.
Figures
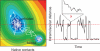
Figure 1
Two-state folding dynamics of an individual protein molecule illustrated as a diffusive process on a two-dimensional free energy surface (left) with a corresponding equilibrium folding/unfolding trajectory (white). An intramolecular distance (corresponding to the distance between a donor and an acceptor fluorophore in a single molecule FRET experiment) is plotted as a function of time (right), showing rapid jumps between folded and unfolded state. An ultimate goal of single molecule experiments is to time-resolve these transitions (expanded scale, top right).
Figure 2
Overview of instrumentation and data reduction in confocal single molecule spectroscopy. The scheme on the right illustrates the main components of a 4-channel confocal single molecule instrument, such as the one commercially available [85], that collects fluorescence photons separated by polarization and wavelength and records their individual arrival times. (a) Sample of a trajectory of detected photons recorded from molecules freely diffusing in solution (in this example Csp_Tm_ in 1.5 M GdmCl [37,38]), where every burst corresponds to an individual molecule traversing the diffraction-limited confocal volume (see upper right of the scheme). (b) 2-dimensional histogram of donor fluorescence lifetime τD versus transfer efficiency E calculated from individual bursts, resulting in subpopulations that can be assigned to the folded and unfolded protein and molecules without active acceptor at E ≈ 0 (shaded in grey). (c) Projection of the histogram onto the E axis. (d) Subpopulation-specific time-correlated single photon counting histograms from donor and acceptor photons from all bursts assigned to unfolded molecules that can be used to extract distance distributions [31,36,38]. (e) Subpopulation-specific donor intensity correlation function, in this case reporting on the nanosecond reconfiguration dynamics of the unfolded protein [58].
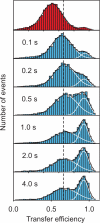
Figure 3
Protein folding kinetics measured with single molecule spectroscopy in a microfluidic mixing device [43]. Starting from Csp_Tm_ unfolded at high denaturant concentration (top), transfer efficiency histograms are measured at different positions along the channel, corresponding to different times after mixing. The fits to Gaussians having the same peak position and width at all times illustrate the redistribution of populations expected for a two-state system after the initial chain collapse.
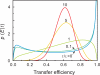
Figure 4
Transfer efficiency distribution p(E | t) for a Gaussian chain with different ratios of observation time t and chain reconfiguration time τr, with t/τr = 0 (dark blue), 0.1 (light blue), 1 (green), 5 (yellow), and 10 (red) (assuming _R_0 = 〈_r_2〉), illustrating the sensitivity of transfer efficiency distributions to chain dynamics. Gopich and Szabo [33] showed that for a Gaussian chain and t >> τr, τr ≈ 10 t _σ_2, with σ2=σobs2−σnoise2, where σobs2 is the observed variance in E, and σnoise2 is the variance due to noise and other effects not dependent on the interdye distance.
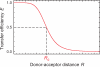
Box. Förster resonance energy transfer
Similar articles
- Direct observation of barrier-limited folding of BBL by single-molecule fluorescence resonance energy transfer.
Huang F, Ying L, Fersht AR. Huang F, et al. Proc Natl Acad Sci U S A. 2009 Sep 22;106(38):16239-44. doi: 10.1073/pnas.0909126106. Epub 2009 Sep 11. Proc Natl Acad Sci U S A. 2009. PMID: 19805287 Free PMC article. - Exploring protein structure and dynamics under denaturing conditions by single-molecule FRET analysis.
Nienhaus GU. Nienhaus GU. Macromol Biosci. 2006 Nov 9;6(11):907-22. doi: 10.1002/mabi.200600158. Macromol Biosci. 2006. PMID: 17099864 - Application of single molecule Förster resonance energy transfer to protein folding.
Schuler B. Schuler B. Methods Mol Biol. 2007;350:115-38. doi: 10.1385/1-59745-189-4:115. Methods Mol Biol. 2007. PMID: 16957321 - Transition Path Times Measured by Single-Molecule Spectroscopy.
Chung HS. Chung HS. J Mol Biol. 2018 Feb 16;430(4):409-423. doi: 10.1016/j.jmb.2017.05.018. Epub 2017 May 25. J Mol Biol. 2018. PMID: 28551335 Free PMC article. Review.
Cited by
- MSMBuilder2: Modeling Conformational Dynamics at the Picosecond to Millisecond Scale.
Beauchamp KA, Bowman GR, Lane TJ, Maibaum L, Haque IS, Pande VS. Beauchamp KA, et al. J Chem Theory Comput. 2011 Oct 11;7(10):3412-3419. doi: 10.1021/ct200463m. J Chem Theory Comput. 2011. PMID: 22125474 Free PMC article. - Quantifying internal friction in unfolded and intrinsically disordered proteins with single-molecule spectroscopy.
Soranno A, Buchli B, Nettels D, Cheng RR, Müller-Späth S, Pfeil SH, Hoffmann A, Lipman EA, Makarov DE, Schuler B. Soranno A, et al. Proc Natl Acad Sci U S A. 2012 Oct 30;109(44):17800-6. doi: 10.1073/pnas.1117368109. Epub 2012 Apr 6. Proc Natl Acad Sci U S A. 2012. PMID: 22492978 Free PMC article. - Exploring the unfolding pathways of protein families using Elastic Network Model.
Kumar R, Dutta S. Kumar R, et al. Sci Rep. 2024 Oct 13;14(1):23905. doi: 10.1038/s41598-024-75436-8. Sci Rep. 2024. PMID: 39397155 Free PMC article. - Disentangling subpopulations in single-molecule FRET and ALEX experiments with photon distribution analysis.
Tomov TE, Tsukanov R, Masoud R, Liber M, Plavner N, Nir E. Tomov TE, et al. Biophys J. 2012 Mar 7;102(5):1163-73. doi: 10.1016/j.bpj.2011.11.4025. Epub 2012 Mar 6. Biophys J. 2012. PMID: 22404939 Free PMC article. - A molecular imaging biosensor detects in vivo protein folding and misfolding.
Sheahan AV, Sekar TV, Chen K, Paulmurugan R, Massoud TF. Sheahan AV, et al. J Mol Med (Berl). 2016 Jul;94(7):799-808. doi: 10.1007/s00109-016-1437-9. Epub 2016 Jun 8. J Mol Med (Berl). 2016. PMID: 27277823
References
- Fisher TE, Oberhauser AF, Carrion-Vazquez M, Marszalek PE, Fernandez JM. The study of protein mechanics with the atomic force microscope. Trends in Biochemical Sciences. 1999;24:379–384. - PubMed
- Zhuang X, Rief M. Single-molecule folding. Curr Opin Struct Biol. 2003;13:88–97. - PubMed
- Bustamante C, Chemla YR, Forde NR, Izhaky D. Mechanical processes in biochemistry. Annu Rev Biochem. 2004;73:705–748. - PubMed
- Forman JR, Clarke J. Mechanical unfolding of proteins: insights into biology, structure and folding. Curr Opin Struct Biol. 2007;17:58–66. - PubMed
- Hummer G, Szabo A. Free energy surfaces from single-molecule force spectroscopy. Accounts Chem Res. 2005;38:504–513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources