Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer - PubMed (original) (raw)
Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer
Jordan M Winter et al. Clin Cancer Res. 2008.
Abstract
Purpose: The role of E-cadherin in carcinogenesis is of great interest, but few studies have examined its relevance to pancreatic carcinoma.
Experimental design: We evaluated E-cadherin protein expression by immunohistochemistry in pancreatobiliary cancers having a noncohesive histologic phenotype (21 undifferentiated adenocarcinomas and 7 signet ring carcinomas), comparing the results with pancreatic cancers having a cohesive phenotype (25 moderately differentiated and 14 poorly differentiated adenocarcinomas).
Results: Twenty of 21 undifferentiated cancers had complete absence of E-cadherin expression, as did two signet ring carcinomas. In contrast, cohesive cancers (n = 39) had E-cadherin labeling at the plasma membrane (P < 0.001). Subsets of cancers were also evaluated for beta-catenin expression. All of the cohesive lesions (n = 28) showed a membranous beta-catenin expression pattern, whereas noncohesive foci (n = 7) were characterized by either cytoplasmic labeling or complete absence of beta-catenin protein expression, suggestive of a deficient zonula adherens in noncohesive cancers. E-cadherin promoter hypermethylation was observed in an undifferentiated pancreatic cancer cell line, MiaPaCa-2, whereas two pancreatic cancer cell lines derived from differentiated lesions lacked any evidence of E-cadherin promoter methylation. No pattern of E-cadherin promoter methylation could be determined in three primary cancers having mixed histologic patterns (contained both cohesive and noncohesive foci). No somatic mutations in E-cadherin were identified in noncohesive pancreatic cancers having inactivated E-cadherin.
Conclusions: Noncohesive pancreatic cancers were characterized by the loss of E-cadherin protein expression. Promoter hypermethylation is a possible mechanism of E-cadherin gene silencing in a subset of these cancers.
Figures
Fig. 1
Representative histologic appearance (H&E) of a moderately differentiated adenocarcinoma (A) and an anaplastic carcinoma (C). The corresponding E-cadherin immunolabeling studies are provided in B and D, respectively. A normal pancreatic duct with membranous E-cadherin protein expression serves as an internal positive control for the noncohesive cancer (anaplastic carcinoma) shown in D.
Fig. 2
Representative β-catenin immunolabeling studies of a moderately differentiated adenocarcinoma (A) and an anaplastic carcinoma (B). β-Catenin protein expression in moderately differentiated (C) and anaplastic (D) components of a heterogeneous cancer is also provided.
Fig. 3
Unmethylated (U)-specific (lanes1, 3, 5, 7, and 9) and methylation (M)-specific (lanes 2, 4, 6, 8, and_10_) PCR amplification of the CpG island in the E-cadherin promoter. Bisulfite-treated water and in vitro – methylated DNA served as negative and positive controls, respectively. A, MiaPaca-2 [undifferentiated (Undiff.) pancreatic cancer], AsPC-1 [differentiated (Diff.) pancreatic cancer], and BxPC-3 (differentiated pancreatic cancer). B, noncohesive (i.e., undifferentiated) and cohesive (i.e., differentiated) foci from three different pancreatic cancers were separated by macrodissecting frozen tumor according to the microscopic appearance of a histologic section from each frozen tumor sample. Neg., negative; Pos., positive.
Fig. 4
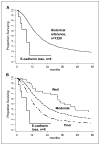
A, Kaplan-Meier survival curves of 9 patients with noncohesive pancreaticobiliary cancers and inactivated E-cadherin (6 anaplastic carcinomas, 1 undifferentiated cancer with osteoclast-like giant cells, and 2 signet ring carcinomas) and 1,229 resected tubuloglandular ductal adenocarcinomas (i.e., cohesive pancreatic cancer; P = 0.002, log-rank test). B, the 1,229 cohesive pancreatic cancers are subgrouped according to histologic grade (well, moderately, and poorly differentiated) and compared with the 9 noncohesive cancers. The P values resulting from comparisons between the nine noncohesive cancers and the subgroups of differentiated pancreatic cancer are P < 0.001, P = 0.0001, and P = 0.03, respectively.
Fig. 5
Proposed model to explain the different noncohesive histologic phenotypes of pancreaticobiliary cancer based on the timing of E-cadherin inactivation during the pancreatic intraepithelialneoplasia (PanIN) adenocarcinoma sequence. OCGT, osteoclast-like giant cell tumor.
Similar articles
- Molecular characterization of undifferentiated-type gastric carcinoma.
Tamura G, Sato K, Akiyama S, Tsuchiya T, Endoh Y, Usuba O, Kimura W, Nishizuka S, Motoyama T. Tamura G, et al. Lab Invest. 2001 Apr;81(4):593-8. doi: 10.1038/labinvest.3780268. Lab Invest. 2001. PMID: 11304579 - Epigenetic inactivation of the E-cadherin gene in eyelid sebaceous gland carcinoma.
Jayaraj P, Sen S, Sharma A, Chosdol K, Kashyap S, Rai A, Pushker N, Bajaj MS, Ghose S. Jayaraj P, et al. Br J Dermatol. 2012 Sep;167(3):583-90. doi: 10.1111/j.1365-2133.2012.10968.x. Epub 2012 Jul 19. Br J Dermatol. 2012. PMID: 22458737 - [CpG island methylation of E-cadherin gene promoter in gastric carcinoma].
Zhou YN, Xu CP, Han B, Ji R. Zhou YN, et al. Ai Zheng. 2005 Oct;24(10):1220-4. Ai Zheng. 2005. PMID: 16219136 Chinese. - Combined loss of E-cadherin and aberrant β-catenin protein expression correlates with a poor prognosis for small intestinal adenocarcinomas.
Lee HJ, Lee OJ, Jang KT, Bae YK, Chung JY, Eom DW, Kim JM, Yu E, Hong SM. Lee HJ, et al. Am J Clin Pathol. 2013 Feb;139(2):167-76. doi: 10.1309/AJCPS54RTFCTHGWX. Am J Clin Pathol. 2013. PMID: 23355201 - Microenvironment-induced restoration of cohesive growth associated with focal activation of P-cadherin expression in lobular breast carcinoma metastatic to the colon.
Gronewold M, Grote I, Bartels S, Christgen H, Kandt LD, Brito MJ, Cserni G, Daemmrich ME, Fogt F, Helmke BM, Ter Hoeve N, Lang-Schwarz C, Vieth M, Wellmann A, Kuehnle E, Kulik U, Riedel G, Reineke-Plaass T, Lehmann U, Koorman T, Derksen PW, Kreipe H, Christgen M. Gronewold M, et al. J Pathol Clin Res. 2024 Mar;10(2):e12361. doi: 10.1002/2056-4538.12361. J Pathol Clin Res. 2024. PMID: 38618992 Free PMC article. Review.
Cited by
- A narrative review on rare types of pancreatic cancer: should they be treated as pancreatic ductal adenocarcinomas?
de Jesus VHF, Donadio MDS, de Brito ÂBC, Gentilli AC. de Jesus VHF, et al. Ther Adv Med Oncol. 2024 Jul 26;16:17588359241265213. doi: 10.1177/17588359241265213. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39072242 Free PMC article. Review. - Comprehensive review of undifferentiated carcinoma of the pancreas: from epidemiology to treatment.
Imaoka H, Ikeda M, Umemoto K, Sunakawa Y, Ueno M, Ueno H, Ozaka M, Kuwahara T, Okano N, Kanai M, Hisano T, Suzuki Y, Asagi A, Shioji K, Todaka A, Tsuji K, Ikezawa K, Miki I, Komatsu Y, Akutsu N, Yamashita T, Okuyama H, Furuse J, Nagano H. Imaoka H, et al. Jpn J Clin Oncol. 2023 Aug 30;53(9):764-773. doi: 10.1093/jjco/hyad062. Jpn J Clin Oncol. 2023. PMID: 37325968 Free PMC article. Review. - Functional biomarkers derived from computed tomography and magnetic resonance imaging differentiate PDAC subgroups and reveal gemcitabine-induced hypo-vascularization.
Heid I, Trajkovic-Arsic M, Lohöfer F, Kaissis G, Harder FN, Mayer M, Topping GJ, Jungmann F, Crone B, Wildgruber M, Karst U, Liotta L, Algül H, Yen HY, Steiger K, Weichert W, Siveke JT, Makowski MR, Braren RF. Heid I, et al. Eur J Nucl Med Mol Imaging. 2022 Dec;50(1):115-129. doi: 10.1007/s00259-022-05930-6. Epub 2022 Sep 8. Eur J Nucl Med Mol Imaging. 2022. PMID: 36074156 Free PMC article. - Epithelial and Mesenchymal Features of Pancreatic Ductal Adenocarcinoma Cell Lines in Two- and Three-Dimensional Cultures.
Shichi Y, Gomi F, Sasaki N, Nonaka K, Arai T, Ishiwata T. Shichi Y, et al. J Pers Med. 2022 May 4;12(5):746. doi: 10.3390/jpm12050746. J Pers Med. 2022. PMID: 35629168 Free PMC article. Review. - microRNA-21 Regulates Stemness in Pancreatic Ductal Adenocarcinoma Cells.
Mortoglou M, Miralles F, Arisan ED, Dart A, Jurcevic S, Lange S, Uysal-Onganer P. Mortoglou M, et al. Int J Mol Sci. 2022 Jan 24;23(3):1275. doi: 10.3390/ijms23031275. Int J Mol Sci. 2022. PMID: 35163198 Free PMC article.
References
- Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21. 1. Science. 1996;271:350–3. - PubMed
- Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002–6. - PubMed
- Xin W, Yun KJ, Ricci F, et al. MAP2K4/MKK4 expression in pancreatic cancer: genetic validation of immunohistochemistry and relationship to disease course. Clin Cancer Res. 2004;10:8516–20. - PubMed
- Bortolasi L, Burgart LJ, Tsiotos GG, Luque-De Leon E, Sarr MG. Adenocarcinoma of the distal bile duct. A clinicopathologic outcome analysis after curative resection. Dig Surg. 2000;17:36–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical