MicroRNAs (miRNAs) in neurodegenerative diseases - PubMed (original) (raw)
Review
MicroRNAs (miRNAs) in neurodegenerative diseases
Peter T Nelson et al. Brain Pathol. 2008 Jan.
Abstract
Aging-related neurodegenerative diseases (NDs) are the culmination of many different genetic and environmental influences. Prior studies have shown that RNAs are pathologically altered during the inexorable course of some NDs. Recent evidence suggests that microRNAs (miRNAs) may be a contributing factor in neurodegeneration. miRNAs are brain-enriched, small ( approximately 22 nucleotides) non-coding RNAs that participate in mRNA translational regulation. Although discovered in the framework of worm development, miRNAs are now appreciated to play a dynamic role in many mammalian brain-related biochemical pathways, including neuroplasticity and stress responses. Research about miRNAs in the context of neurodegeneration is accumulating rapidly, and the goal of this review is to provide perspective for these new data that may be helpful to specialists in either field. An overview is provided about the normal functions for miRNAs, including some of the newer concepts related to the human brain. Recently published studies pertaining to the roles of miRNAs in NDs--including Alzheimer's disease, Parkinson's disease and triplet repeat disorders-are described. Finally, a discussion is included with theoretical syntheses and possible future directions in exploring the nexus between miRNA and ND research.
Figures
Figure 1
In the mammalian brain as elsewhere, miRNAs subserve multiple fundamental biological roles. Many miRNAs are thought to interact with thousands of different targets. Post‐transcriptional RNA editing may change mRNA target specifity. References for the different hypothesized functions of miRNAs are given in the text.
Figure 2
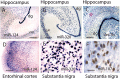
In situ hybridization (ISH) from normal human brains for miRNAs gives an idea of how miRNAs are expressed in neuroanatomical areas that are vulnerable to NDs. A–C show hippocampus. A,B shows miRNAs (miR‐124 and miR‐320) that are expressed predominantly in neurons. By contrast, let‐7b is expressed in both neurons and glia, so cells outside the neuronal layers (such as the molecular layer, pink star, of the dentate granules, dg) are stained. D shows that cells of the superficial layer of entorhinal cortex are also stained avidly using a miR‐124 probe. In the substantia nigra, miR‐320 labels the pigment‐containing cells (E), but miR‐107 only labels them barely if at all (F). Scale bars: A–D = 500 µM; E = 125 µM; F = 62.5 µM. ISH was performed according to the protocol previously described (89) except fixed brain was cut with a freezing microtome.
Figure 3
Not only does a given miRNA recognize many mRNA targets, but the 3′ untranslated region (3′UTR) of many mRNAs are also the potential targets for many different miRNAs. Here are two examples that are relevant to NDs, the beta‐amyloid precursor protein (APP) and alpha‐synuclein (SNCA). miRNA target predictions are from the miRBASE registry (
http://microrna.sanger.ac.uk/targets/v4/
), and have not been validated. APP is predicted to be recognized by the products of 41 different miRNA genes, and SNCA is predicted to be recognized by that of 12.
Figure 4
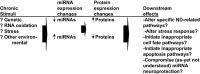
A schematic diagram about some roles miRNAs may play in NDs. The brains of persons at risk for NDs are subjected to chronic stimuli that can perturb specific and/or global miRNA expression. Either the aberrant stimulation or inhibition of miRNA expression may be associated with pathways involved in NDs or neuroprotection.
Similar articles
- Causes and Consequences of MicroRNA Dysregulation in Neurodegenerative Diseases.
Tan L, Yu JT, Tan L. Tan L, et al. Mol Neurobiol. 2015;51(3):1249-62. doi: 10.1007/s12035-014-8803-9. Epub 2014 Jun 29. Mol Neurobiol. 2015. PMID: 24973986 Review. - MicroRNAs in glaucoma and neurodegenerative diseases.
Molasy M, Walczak A, Szaflik J, Szaflik JP, Majsterek I. Molasy M, et al. J Hum Genet. 2017 Jan;62(1):105-112. doi: 10.1038/jhg.2016.91. Epub 2016 Jul 14. J Hum Genet. 2017. PMID: 27412874 Review. - New insights into the role of microRNAs and long noncoding RNAs in most common neurodegenerative diseases.
Maniati MS, Maniati M, Yousefi T, Ahmadi-Ahangar A, Tehrani SS. Maniati MS, et al. J Cell Biochem. 2019 Jun;120(6):8908-8918. doi: 10.1002/jcb.28361. Epub 2019 Jan 20. J Cell Biochem. 2019. PMID: 30663117 Review. - MicroRNAs Dysregulation and Mitochondrial Dysfunction in Neurodegenerative Diseases.
Catanesi M, d'Angelo M, Tupone MG, Benedetti E, Giordano A, Castelli V, Cimini A. Catanesi M, et al. Int J Mol Sci. 2020 Aug 20;21(17):5986. doi: 10.3390/ijms21175986. Int J Mol Sci. 2020. PMID: 32825273 Free PMC article. Review. - Targeting MicroRNAs in Prevention and Treatment of Neurodegenerative Disorders.
Gupta S, Verma S, Mantri S, Berman NE, Sandhir R. Gupta S, et al. Drug Dev Res. 2015 Nov;76(7):397-418. doi: 10.1002/ddr.21277. Epub 2015 Sep 11. Drug Dev Res. 2015. PMID: 26359796 Review.
Cited by
- MicroRNAs and deregulated gene expression networks in neurodegeneration.
Sonntag KC. Sonntag KC. Brain Res. 2010 Jun 18;1338:48-57. doi: 10.1016/j.brainres.2010.03.106. Epub 2010 Apr 7. Brain Res. 2010. PMID: 20380815 Free PMC article. Review. - Expression profile of MicroRNAs in young stroke patients.
Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Tan KS, et al. PLoS One. 2009 Nov 2;4(11):e7689. doi: 10.1371/journal.pone.0007689. PLoS One. 2009. PMID: 19888324 Free PMC article. - MicroRNA in Situ Hybridization in the Human Entorhinal and Transentorhinal Cortex.
Nelson PT, Dimayuga J, Wilfred BR. Nelson PT, et al. Front Hum Neurosci. 2010 Feb 22;4:7. doi: 10.3389/neuro.09.007.2010. eCollection 2010. Front Hum Neurosci. 2010. PMID: 20204141 Free PMC article. - Identification of glucose-regulated miRNAs from pancreatic {beta} cells reveals a role for miR-30d in insulin transcription.
Tang X, Muniappan L, Tang G, Ozcan S. Tang X, et al. RNA. 2009 Feb;15(2):287-93. doi: 10.1261/rna.1211209. Epub 2008 Dec 18. RNA. 2009. PMID: 19096044 Free PMC article.
References
- Ambros V (2003) MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell 113:673–676. - PubMed
- Arendt T (2005) Alzheimer's disease as a disorder of dynamic brain self‐organization. Prog Brain Res 147:355–78. - PubMed
Note added in proof
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical