TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway - PubMed (original) (raw)
TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway
Qun-Ying Lei et al. Mol Cell Biol. 2008 Apr.
Abstract
TAZ is a WW domain containing a transcription coactivator that modulates mesenchymal differentiation and development of multiple organs. In this study, we show that TAZ is phosphorylated by the Lats tumor suppressor kinase, a key component of the Hippo pathway, whose alterations result in organ and tissue hypertrophy in Drosophila and contribute to tumorigenesis in humans. Lats phosphorylates TAZ on several serine residues in the conserved HXRXXS motif and creates 14-3-3 binding sites, leading to cytoplasmic retention and functional inactivation of TAZ. Ectopic expression of TAZ stimulates cell proliferation, reduces cell contact inhibition, and promotes epithelial-mesenchymal transition (EMT). Elimination of the Lats phosphorylation sites results in a constitutively active TAZ, enhancing the activity of TAZ in promoting cell proliferation and EMT. Our results elucidate a molecular mechanism for TAZ regulation and indicate a potential function of TAZ as an important target of the Hippo pathway in regulating cell proliferation tumorigenesis.
Figures
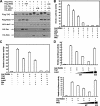
FIG. 1.
The transcription coactivator activity of TAZ is inhibited by the Hippo pathway. (A) The cotransfection of Mst2 and Lats2 decreases TAZ electrophoretic mobility. Flag-TAZ was cotransfected with the indicated plasmids into BOSC cells (a cell line derived from HEK 293), and Western blotting was employed to examine TAZ mobility. (B) TAZ activity is repressed by Mst2 and Lats2. The 5× GAL4 UAS-luciferase reporter and GAL4-TEAD4 were transfected into BOCS cells, with the indicated plasmids. Renilla luciferase plasmid was also cotransfected as an internal control. Firefly luciferase activity was measured and normalized to Renilla luciferase activity. Data are representative of three independent experiments. The coexpression of the Hippo pathway components Mst2, Sav, Lats2, and Mob inhibited TAZ activity. (C) TAZ activity is inhibited by human Ex and Mer. The experiments shown are similar to those shown in panel B. Both Ex and Mer cause a significant inhibition of the TAZ reporter. (D) Kinase activity is required for Mst2 and Lats2 to inhibit TAZ. Increasing amounts of wild-type Lats2 (upper panel) or Mst2 (lower panel) were cotransfected with the TAZ reporter. Both Lats2 and Mst2 show a dose-dependent inhibition of the TAZ reporter. In contrast, the kinase-inactive mutants (K/R) of both Lats2 and Mst2 cannot inhibit the TAZ reporter.
FIG. 2.
Lats2 inhibits the function of TAZ via phosphorylation. (A) TAZ contains four HXRXXS motifs as the putative Lats phosphorylation sites. The yeast Dbf2 optimal target sequence was aligned with the four HXRXXS motifs of human TAZ. (B) The putative HXRXXS motifs in TAZ are required for a mobility shift by Lats2 and Mst2. The wild type (WT) or the mutant Flag-TAZ was cotransfected with HA-Mst2 and HA-Last2, as indicated. The TAZ mobility shift was examined by Western blotting. The 4SA mutation denotes the quadruple mutation of all four putative phosphorylation sites. As indicated, treatment with lambda phosphatase abolished the mobility shift by Lats2 and Mst2 cotransfection, indicating that the mobility shift is due to phosphorylation. (C) The S89A and S4A TAZ phosphorylation mutants are resistant to inhibition by Mst2 and Lats2. The S89A mutation and the phosphorylation mimic S89D mutant made no difference in the inhibition by Mst2 and Lats2. The reporter assay was performed similar to that shown in panel A. The inhibition change (_n_-fold) for each mutant is shown at the top of this panel. (D) The coexpression of Mst2 and Lats2 increases TAZ Ser89 phosphorylation. Lats2K/R dramatically decreases TAZ Ser89 phosphorylation. Flag-TAZ was cotransfected with Lats2, Mst2, or kinase-dead Lats2K/R into 293T cells, as indicated. Flag-TAZ was immunoprecipitated, and phosphorylation of Ser89 was detected by pYAP(Ser127) antibody, which can recognize the Ser89 phosphorylation site because of sequence conservation. Lambda phosphatase treatment and the TAZS89A mutant abolished the recognition of phosphoTAZ by the anti-phosphoYAP antibody. This result confirms the specificity of the phosphoYAP antibody. (E) In vitro phosphorylation of TAZ by Lats2. HA-Lats2 or Flag-Mst2 was immunoprecipitated from transfected 293T cells. An in vitro kinase assay was performed using purified His-TAZ as a substrate. Phosphorylation of TAZ was detected by pYAP(S127) antibody. His-TAZ input was shown by Coomassie blue staining. (F) A knockdown of Lats decreases TAZ Ser89 phosphorylation. HeLa cells cotransfected HA-TAZ with small interfering (siRNA) for Lats1 and Lats2, as indicated. Phosphorylation and protein levels of TAZ were determined by Western blotting. A knockdown of Lats was verified by quantitative PCR (data not shown).
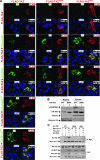
FIG. 3.
Phosphorylation increases TAZ cytoplasmic localization and 14-3-3 association. (A) Phosphorylation is important for TAZ nuclear exclusion caused by Lats2 and Mst2. Flag-TAZ (wild type and S89A and 4SA mutants) was cotransfected with HA-Lats2 or HA-Mst2, as indicated. Immunofluorescence using anti-Flag antibody (red) and HA antibody (green) was performed. DNA was stained with 4′,6′-diamidino-2-phenylindole (DAPI) (blue). The merged figures are shown in the lower right corner of each panel. (B) The mutation of Ser89 increases nuclear TAZ. HA-TAZ and the S89A mutant were cotransfected with Mst2 and Lats2 into HEK293 cells. Cytoplasmic and nuclear fractions were separated and analyzed by Western blotting. WT, wild type. (C) Flag-TAZ was cotransfected with Myc 14-3-3 and other indicated plasmids into 293T cells. Myc 14-3-3 was immunoprecipitated and coimmunoprecipitated. Flag-TAZ was probed with Flag antibody.
FIG. 4.
TAZ stimulates cell proliferation. (A) TAZ promotes cell growth. Growth curves of MCF10A cells stably expressing pBabe-vector, -TAZ, and -TAZ4SA were determined. (B) TAZ increases the proliferation of MCF10A cells. A synchronized parental MCF10A and pBabe-vector, -TAZ, and -TAZ4SA overexpressing MCF10A cells were labeled with BrdU (15 μM for 30 min), followed by staining with anti-BrdU and propidium iodide for flow cytometric analysis.
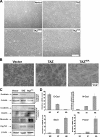
FIG. 5.
TAZ induces the EMT of MCF10A cells. (A) TAZ induces a morphology change in MCF10A cells. Phase-contrast images of MCF10A cells expressing vector, TAZ, TAZS89A, and TAZ4SA are shown. WT, wild type. (B) TAZ alters the actin organization. Cells were stained with rhodamine-conjugated phalloidin. (C) TAZ causes EMT in MCF10A cells. Cell lysates from MCF10A cells expressing vector, TAZ, and TAZ4SA were separated and probed with antibodies for epithelial markers and mesenchymal markers as indicated. (D) TAZ decreases E-cadherin and increases N-cadherin expression through the activation of EMT transcription. Total RNA was extracted from MCF10A-vector, -TAZ, and -TAZ4SA cells, and quantitative PCR was performed to determine E-cadherin and N-cadherin expression (upper panel) and Foxc2 and Snail expression (lower panel). All data are normalized to GAPDH.
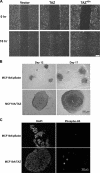
FIG. 6.
TAZ enhances cell migration and acinar growth in MCF10A cells. (A) TAZ overexpression promotes cell migration. MCF10A cells expressing vector, TAZ wild type (WT), and TAZ4SA (4SA) were analyzed for migration by a wound-healing assay. (B) TAZ stimulates acinar growth. MCF10A and derivative cells were cultured in the Matrigel. The acini formed at day 12 and 17 are shown. Note the large acini with lumen (L) formed by MCF-10A/TAZ. (C) TAZ promotes MCF10A proliferation in Matrigel culture. MCF10A and derivative cells were cultured in Matrigel and acini formed at day 17, fixed, paraffin embedded, and immunostained with antibody to PH3.
Similar articles
- Ski regulates Hippo and TAZ signaling to suppress breast cancer progression.
Rashidian J, Le Scolan E, Ji X, Zhu Q, Mulvihill MM, Nomura D, Luo K. Rashidian J, et al. Sci Signal. 2015 Feb 10;8(363):ra14. doi: 10.1126/scisignal.2005735. Sci Signal. 2015. PMID: 25670202 Free PMC article. - Mammalian NDR/LATS protein kinases in hippo tumor suppressor signaling.
Hergovich A, Hemmings BA. Hergovich A, et al. Biofactors. 2009 Jul-Aug;35(4):338-45. doi: 10.1002/biof.47. Biofactors. 2009. PMID: 19484742 Review. - Arhgef7 promotes activation of the Hippo pathway core kinase Lats.
Heidary Arash E, Song KM, Song S, Shiban A, Attisano L. Heidary Arash E, et al. EMBO J. 2014 Dec 17;33(24):2997-3011. doi: 10.15252/embj.201490230. Epub 2014 Nov 25. EMBO J. 2014. PMID: 25425573 Free PMC article. - Tumor suppressor LATS1 is a negative regulator of oncogene YAP.
Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Hao Y, et al. J Biol Chem. 2008 Feb 29;283(9):5496-509. doi: 10.1074/jbc.M709037200. Epub 2007 Dec 24. J Biol Chem. 2008. PMID: 18158288 - YAP and TAZ in epithelial stem cells: A sensor for cell polarity, mechanical forces and tissue damage.
Elbediwy A, Vincent-Mistiaen ZI, Thompson BJ. Elbediwy A, et al. Bioessays. 2016 Jul;38(7):644-53. doi: 10.1002/bies.201600037. Epub 2016 May 13. Bioessays. 2016. PMID: 27173018 Free PMC article. Review.
Cited by
- Quit your YAPing: a new target for cancer therapy.
Stanger BZ. Stanger BZ. Genes Dev. 2012 Jun 15;26(12):1263-7. doi: 10.1101/gad.196501.112. Genes Dev. 2012. PMID: 22713867 Free PMC article. - Inhibitory mechanism of FAT4 gene expression in response to actin dynamics during Src-induced carcinogenesis.
Ito T, Taniguchi H, Fukagai K, Okamuro S, Kobayashi A. Ito T, et al. PLoS One. 2015 Feb 13;10(2):e0118336. doi: 10.1371/journal.pone.0118336. eCollection 2015. PLoS One. 2015. PMID: 25679223 Free PMC article. - Exploring the interaction between extracellular matrix components in a 3D organoid disease model to replicate the pathophysiology of breast cancer.
Bhattacharya A, Alam K, Roy NS, Kaur K, Kaity S, Ravichandiran V, Roy S. Bhattacharya A, et al. J Exp Clin Cancer Res. 2023 Dec 16;42(1):343. doi: 10.1186/s13046-023-02926-4. J Exp Clin Cancer Res. 2023. PMID: 38102637 Free PMC article. Review. - Heterogeneity of Hippo signalling activity in different histopathologic subtypes of renal cell carcinoma.
Duong NX, Le MK, Kondo T, Mitsui T. Duong NX, et al. J Cell Mol Med. 2023 Jan;27(1):66-75. doi: 10.1111/jcmm.17632. Epub 2022 Dec 7. J Cell Mol Med. 2023. PMID: 36478130 Free PMC article. - TAZ suppresses NFAT5 activity through tyrosine phosphorylation.
Jang EJ, Jeong H, Han KH, Kwon HM, Hong JH, Hwang ES. Jang EJ, et al. Mol Cell Biol. 2012 Dec;32(24):4925-32. doi: 10.1128/MCB.00392-12. Epub 2012 Oct 8. Mol Cell Biol. 2012. PMID: 23045390 Free PMC article.
References
- Basu, S., N. F. Totty, M. S. Irwin, M. Sudol, and J. Downward. 2003. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol. Cell 1111-23. - PubMed
- Cho, E., Y. Feng, C. Rauskolb, S. Maitra, R. Fehon, and K. D. Irvine. 2006. Delineation of a Fat tumor suppressor pathway. Nat. Genet. 381142-1150. - PubMed
- Dan, I., N. M. Watanabe, and A. Kusumi. 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11220-230. - PubMed
- Debnath, J., S. K. Muthuswamy, and J. S. Brugge. 2003. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30256-268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous