The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP - PubMed (original) (raw)
The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP
Cai-Hong Yun et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Lung cancers caused by activating mutations in the epidermal growth factor receptor (EGFR) are initially responsive to small molecule tyrosine kinase inhibitors (TKIs), but the efficacy of these agents is often limited because of the emergence of drug resistance conferred by a second mutation, T790M. Threonine 790 is the "gatekeeper" residue, an important determinant of inhibitor specificity in the ATP binding pocket. The T790M mutation has been thought to cause resistance by sterically blocking binding of TKIs such as gefitinib and erlotinib, but this explanation is difficult to reconcile with the fact that it remains sensitive to structurally similar irreversible inhibitors. Here, we show by using a direct binding assay that T790M mutants retain low-nanomolar affinity for gefitinib. Furthermore, we show that the T790M mutation activates WT EGFR and that introduction of the T790M mutation increases the ATP affinity of the oncogenic L858R mutant by more than an order of magnitude. The increased ATP affinity is the primary mechanism by which the T790M mutation confers drug resistance. Crystallographic analysis of the T790M mutant shows how it can adapt to accommodate tight binding of diverse inhibitors, including the irreversible inhibitor HKI-272, and also suggests a structural mechanism for catalytic activation. We conclude that the T790M mutation is a "generic" resistance mutation that will reduce the potency of any ATP-competitive kinase inhibitor and that irreversible inhibitors overcome this resistance simply through covalent binding, not as a result of an alternative binding mode.
Conflict of interest statement
Conflict of interest statement: M.J.E. and M.M. are consultants for and receive research funding from Novartis Institutes for Biomedical Research.
Figures
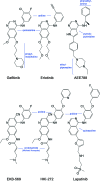
Fig. 1.
Chemical structures of selected EGFR inhibitors. All compounds are drawn in a consistent orientation and conformation that reflects their approximate binding mode in the EGFR kinase. HKI-272 and EKB-569 are examples of irreversible inhibitors. Lapatinib and HKI-272 are thought to require the inactive conformation of EGFR for binding because of their additional aniline substitutions.
Fig. 2.
Crystal structures of the EGFR T790M mutant show that inhibitors are readily accommodated in the active and inactive conformations of the kinase. (A) Superposition of EGFR T790M/AEE788 complex (yellow) and WT/AEE788 complex [light blue; drawn from PDB ID code 2J6M (8)]. Dashed lines indicate hydrogen bonds to the kinase hinge region that are preserved in both complexes. The location of the T790M mutation is indicated. (B) Superposition of EGFR T790M/AEE788 complex (yellow) and apo-T790M structure (green). Note the alternate side-chain conformation of Met-790 in the presence of the inhibitor. (C) Crystal structure of HKI-272 in complex with the T790M mutant. The kinase adopts an inactive conformation, with the C-helix displaced. A covalent bond is formed between Cys-797 and the crotonamide Michael acceptor of HKI-272. (D) The structure of the T790M mutant in complex with HKI-272 (yellow) is superimposed on the structure of the WT EGFR kinase in complex with Lapatinib [light blue; drawn from PDB ID code 1XKK (32)]. In both structures, the kinase adopts the same inactive conformation and the inhibitors bind in a similar manner, with a single hydrogen bond to the hinge (dashed lines) and with their aniline substituents extending into the enlarged hydrophobic pocket that is characteristic of the inactive conformation.
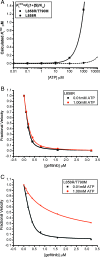
Fig. 3.
The drug resistance of T790M secondary mutation is manifested only at cellular concentrations of ATP. (A) The calculated _K_iapp for the L858R single mutant and L858R/T790M double mutant are plotted versus ATP concentration, using experimentally measured values for the _K_m[ATP] (Table 2) and setting _K_i = _K_d as measured (Table 1). Note the expected loss of potency of the double mutant (solid line) as ATP concentrations approach cellular levels (≈1 mM). (B) Inhibition of L858R mutant EGFR kinase by gefitinib in the presence of 10 μM (black squares) or 1.0 mM ATP (red circles). (C) Inhibition of the L858R/T790M double mutant EGFR by gefitinib in the presence of 10 μM (black squares) or 1.0 mM ATP (red circles). In B and C, the in vitro kinase activity of the indicated EGFR mutant was measured in the presence of the indicated concentrations of gefitinib by using an EGFR Tyr-1173 autophosphorylation site peptide (ENAEYLRVA) as substrate.
Similar articles
- The T790M "gatekeeper" mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor.
Godin-Heymann N, Ulkus L, Brannigan BW, McDermott U, Lamb J, Maheswaran S, Settleman J, Haber DA. Godin-Heymann N, et al. Mol Cancer Ther. 2008 Apr;7(4):874-9. doi: 10.1158/1535-7163.MCT-07-2387. Mol Cancer Ther. 2008. PMID: 18413800 - Structural insight into the binding mechanism of ATP to EGFR and L858R, and T790M and L858R/T790 mutants.
Saldaña-Rivera L, Bello M, Méndez-Luna D. Saldaña-Rivera L, et al. J Biomol Struct Dyn. 2019 Oct;37(17):4671-4684. doi: 10.1080/07391102.2018.1558112. Epub 2019 Jan 11. J Biomol Struct Dyn. 2019. PMID: 30558477 - Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors.
Jia Y, Yun CH, Park E, Ercan D, Manuia M, Juarez J, Xu C, Rhee K, Chen T, Zhang H, Palakurthi S, Jang J, Lelais G, DiDonato M, Bursulaya B, Michellys PY, Epple R, Marsilje TH, McNeill M, Lu W, Harris J, Bender S, Wong KK, Jänne PA, Eck MJ. Jia Y, et al. Nature. 2016 Jun 2;534(7605):129-32. doi: 10.1038/nature17960. Epub 2016 May 25. Nature. 2016. PMID: 27251290 Free PMC article. - Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers.
Roskoski R Jr. Roskoski R Jr. Pharmacol Res. 2019 Jan;139:395-411. doi: 10.1016/j.phrs.2018.11.014. Epub 2018 Nov 27. Pharmacol Res. 2019. PMID: 30500458 Review. - Challenges of detecting EGFR T790M in gefitinib/erlotinib-resistant tumours.
Jänne PA. Jänne PA. Lung Cancer. 2008 Jun;60 Suppl 2:S3-9. doi: 10.1016/S0169-5002(08)70099-0. Lung Cancer. 2008. PMID: 18513582 Review.
Cited by
- Detailed characterization of combination treatment with MET inhibitor plus EGFR inhibitor in _EGFR_-mutant and _MET_-amplified non-small cell lung cancer.
Lee Y, Park SY, Lee GK, Lim HJ, Choi YR, Kim J, Han JY. Lee Y, et al. Transl Lung Cancer Res. 2024 Oct 31;13(10):2511-2523. doi: 10.21037/tlcr-24-273. Epub 2024 Oct 11. Transl Lung Cancer Res. 2024. PMID: 39507027 Free PMC article. - Design, in silico studies and biological evaluation of novel chalcones tethered triazolo[3,4-a]isoquinoline as EGFR inhibitors targeting resistance in non-small cell lung cancer.
Abdelaal N, Ragheb MA, Hassaneen HM, Elzayat EM, Abdelhamid IA. Abdelaal N, et al. Sci Rep. 2024 Nov 4;14(1):26647. doi: 10.1038/s41598-024-76459-x. Sci Rep. 2024. PMID: 39496648 Free PMC article. - Inhibitory effect of 1,4,5,6-tetrahydroxy-7,8-diprenylxanthone against NSCLC with L858R/T790M/C797S mutant EGFR.
Wang J, Wang Y, Zhang S, Qu Y, Zhang R, Wang X, Sheng J, Sun P. Wang J, et al. Sci Rep. 2024 Nov 4;14(1):26549. doi: 10.1038/s41598-024-78146-3. Sci Rep. 2024. PMID: 39489821 Free PMC article. - Biochemical analysis of EGFR exon20 insertion variants insASV and insSVD and their inhibitor sensitivity.
Zhao H, Beyett TS, Jiang J, Rana JK, Schaeffner IK, Santana J, Jänne PA, Eck MJ. Zhao H, et al. Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2417144121. doi: 10.1073/pnas.2417144121. Epub 2024 Oct 29. Proc Natl Acad Sci U S A. 2024. PMID: 39471218 Free PMC article. - Current Drug Resistance Mechanisms and Treatment Options in Gastrointestinal Stromal Tumors: Summary and Update.
He C, Wang Z, Yu J, Mao S, Xiang X. He C, et al. Curr Treat Options Oncol. 2024 Nov;25(11):1390-1405. doi: 10.1007/s11864-024-01272-7. Epub 2024 Oct 23. Curr Treat Options Oncol. 2024. PMID: 39441520 Free PMC article. Review.
References
- Paez JG, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
- Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
- Johnson BE, Janne PA. Epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Cancer Res. 2005;65:7525–7529. - PubMed
- Gazdar AF, Shigematsu H, Herz J, Minna JD. Mutations and addiction to EGFR: The Achilles “heal” of lung cancers? Trends Mol Med. 2004;10:481–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Research Materials
Miscellaneous