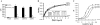Mouse EP3 alpha, beta, and gamma receptor variants reduce tumor cell proliferation and tumorigenesis in vivo - PubMed (original) (raw)
Mouse EP3 alpha, beta, and gamma receptor variants reduce tumor cell proliferation and tumorigenesis in vivo
Ines M Macias-Perez et al. J Biol Chem. 2008.
Abstract
Prostaglandin E(2), which exerts its functions by binding to four G protein-coupled receptors (EP1-4), is implicated in tumorigenesis. Among the four E-prostanoid (EP) receptors, EP3 is unique in that it exists as alternatively spliced variants, characterized by differences in the cytoplasmic C-terminal tail. Although three EP3 variants, alpha, beta, and gamma, have been described in mice, their functional significance in regulating tumorigenesis is unknown. In this study we provide evidence that expressing murine EP3 alpha, beta, and gamma receptor variants in tumor cells reduces to the same degree their tumorigenic potential in vivo. In addition, activation of each of the three mEP3 variants induces enhanced cell-cell contact and reduces cell proliferation in vitro in a Rho-dependent manner. Finally, we demonstrate that EP3-mediated RhoA activation requires the engagement of the heterotrimeric G protein G(12). Thus, our study provides strong evidence that selective activation of each of the three variants of the EP3 receptor suppresses tumor cell function by activating a G(12)-RhoA pathway.
Figures
FIGURE 1.
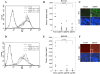
The mEP3 receptor variants inhibit growth in vivo.A, flow cytometry of stably transfected HCT116 cells with mEP3 receptor variants labeled with anti-Myc antibody. mEP3 receptor expression is displayed by a shift in mean fluorescent intensity compared with empty vector-transfected HCT116 cells. The values represent the means ± S.D. of mean fluorescent intensity of three independent experiments. B, 1 × 106 empty vector-transfected and mEP3-expressing HCT116 cells were injected subcutaneously into nude mice (n = 10/cell type). 35 days later the mice were sacrificed, and tumor volume was evaluated as described under “Experimental Procedures.” The open squares represent the volume of single tumors, whereas the bars represent the means. C, frozen sections of tumors derived from the tumors indicated (only mEP3γ shown) were stained with anti-Myc antibody to confirm the in vivo expression of the mEP3 receptor variants.D, flow cytometric analysis of stably transfected HEK293 cells with mEP3 receptor variants was performed as described in A. E, 1 × 106 empty vector-transfected and mEP3-expressing HEK293 cells were injected subcutaneously into nude mice (n = 10/cell type). 35 days later mice were sacrificed, and tumor volume was evaluated as described under “Experimental Procedures.” The open circles represent the volume of single tumors, whereas the bars represent the mean.F, frozen sections of the tumors indicated (only mEP3γ shown) were stained with anti-Myc antibody to confirm the in vivo expression of the mEP3 receptor variants. DAPI, 4′,6′-diamino-2-phenylindole.
FIGURE 2.
Characterization of the mEP3 receptor variants. A, one-point ligand binding assay on 50-μg membranes from empty vector-transfected and mEP3-expressing HEK293. The bars and error bars are the means ± S.D. of triplicate samples. *, significant differences (p < 0.05) between vehicle-treated and PGE2-treated membranes; #, significant differences (p < 0.05) between PGE2-treated and PGE2+Sulprostone-treated membranes. B, saturation isotherm analysis of [3H]PGE2 binding to empty vector-transfected and mEP3-expressing HEK293 cells. The membranes (5 μg) were incubated with [3H]PGE2 at the concentrations indicated in the absence (total binding) or presence (nonspecific binding) of 10 μ
m
PGE2, as described under “Experimental Procedures.” Specific binding (total-nonspecific) is shown. The values in the_inset_ represent the means ± S.D. of one representative experiment performed in triplicate. C, dose response of M&B28767-induced intracellular calcium mobilization was evaluated in empty vector-transfected and mEP3-expressing HEK293 cells as described under “Experimental Procedures.”
FIGURE 3.
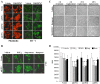
Activation of the mEP3 receptor variants enhances cell-cell contact and reduces growth in vitro. A, empty vector-transfected and mEP3-expressing HEK293 cells were treated with vehicle or 0.1 μ
m
M&B28767. After 4 h, the cells were stained with rhodamine-phalloidin and anti-ZO-1 antibody to visualize the cytoskeleton and tight junctions, respectively. Scale bar, 20 μm. The images are representative of three independent experiments. B, empty vector-transfected and mEP3-expressing HEK293 cells (mEP3γ shown only) were treated with vehicle or 0.1 μ
m
PGE2, sulprostone, or butaprost. After 4 h the cells were stained with anti-ZO1 antibody to visualize tight junctions. Scale bar, 20 μm. The images are representative of three independent experiments. C, the cells indicated were treated with 0.1 μ
m
M&B28767, butaprost or vehicle. After 4 h the medium was replaced with medium lacking prostanoid, and the cells were imaged 4, 24, and 48 h after medium replacement. The images shown are representative of three independent experiments. D,[3H]thymidine incorporation on empty vector-transfected and mEP3-expressing HEK293 cells treated with vehicle, PGE2, M&B28767 (MB), sulprostone (Sulp), or butaprost (Buta) was evaluated as described under “Experimental Procedures.” The bars and error bars represent the means ± S.D. of one experiments performed in quadruplicate. *, significant differences (p < 0.05) between vehicle-treated and prostanoid-treated cells.
FIGURE 4.
The mEP3 receptor variants activate ERK and Rho. _A_-C, serum-starved cells were treated with vehicle or 0.1 μ
m
M&B28767 for 15 min, and the levels of phosphorylated p38 MAPK, Akt, and ERK as well as total p38 MAPK, Akt, and ERK were analyzed by Western blot in 30 μg of total cell lysates. FCS represents serum cells stimulated for 15 min with 10% fetal calf serum as positive control.D, Rho pulldown assay performed on cells lysates of serum-starved cells treated with vehicle or 0.1 μ
m
M&B28767 for 15 min. Bound GTP-Rho was detected by immunoblotting with anti-Rho antibody (top). The total cell lysates were used to detect the levels of total Rho (bottom).
FIGURE 5.
mEP3-induced morphological change and growth inhibition are mediated by Rho. A, mEP3-expressing HEK293 cells (mEP3γ shown only) were serum-starved for 12 h in the presence or absence of PD-98059 (5 μ
m
), wortmannin (Wort, 100 n
m
), isobutylmethylxanthine (IBMX, 0.5 m
m
), 5 μ
m
BAPTA/AM (5 μ
m
), or Y-27632 (5 μ
m
) or transfected for 48 h with the C3 toxin expression plasmid. The cells were subsequently treated with vehicle (Veh) or 0.1 μ
m
M&B28767 (MB), and their morphology was evaluated 4 h after treatment. The images are representative of three independent experiments. B, Rho pulldown assay was performed on cells lysates of serum-starved empty vector-transfected and mEP3-expressing HEK293 (mEP3γ shown only) cells treated as indicated. GTP-Rho was detected by immunoblotting with anti-Rho antibody (top). Total cell lysates were used to detect the levels of total Rho (bottom). C,[3H]thymidine incorporation assay on empty vector-transfected and mEP3-expressing HEK293 cells incubated in the presence or absence of 10 μ
m
Y-27632, 0.1 μ
m
PGE2, and 0.1 μ
m
M&B28767 (MB) was evaluated as described under “Experimental Procedures.” The bars and error bars represent the means ± S.D. of a representative experiment performed in quadruplicates. *, significant differences (p < 0.05) between vehicle and PGE2- or MB-treated cells. **, significant differences (p < 0.05) between MB- and MB+Y-27632 or PGE2- and PGE2+Y-27632 treated cells.
FIGURE 6.
Activated Rho, G12 and G13 induce a morphological change characterized by enhanced cell-cell contact. A, the cells indicated were transfected with empty vector or dominant active Rho (DARho), and the levels of Rho were evaluated 48 h after transfection by Western blot analysis in 30 μg of total cell lysates. The membranes were subsequently incubated with anti-FAK antibody to verify equal loading.B, 48 h empty vector- or DARho-transfected cells were treated with vehicle or 0.1 μ
m
M&B28767, and their morphology was evaluated 4 h after treatment. The images are representative of three independent experiments. C, the cells indicated were transfected with only or dominant active Gi2, Gα12, or Gα13, and the levels of these G proteinα subunits were evaluated 48 h after transfection by Western blot analysis in 30 μg of total cell lysates. The membranes were subsequently incubated with anti-FAK antibody to verify equal loading. D, the cells indicated were transfected with the constructs indicated above. 48 h later cell morphology was evaluated in 4-h vehicle-treated cells. The images are representative of three independent experiments.
FIGURE 7.
The mEP3-induced morphological change is mediated by G12. A, mEP3-expressing HEK293 (mEP3γ shown only) were transfected with empty vector or dominant negative Gi, Gα12, or Gα13, and the levels of these G protein α subunits were evaluated 48 h after transfection by Western blot analysis in 30 μg of total cell lysates. The membranes were subsequently incubated with anti-FAK antibody to verify equal loading. B, mEP3-expressing HEK293 cells (mEP3γ shown only) were transfected with the constructs indicated above. Forty-eight h later the cells were either treated with vehicle or 0.1 μ
m
M&B28767, and their morphology was evaluated 4 h after treatment. The images are representative of three independent experiments.
Similar articles
- Prostaglandin receptors: advances in the study of EP3 receptor signaling.
Hatae N, Sugimoto Y, Ichikawa A. Hatae N, et al. J Biochem. 2002 Jun;131(6):781-4. doi: 10.1093/oxfordjournals.jbchem.a003165. J Biochem. 2002. PMID: 12038972 Review. - Opposing effects of prostaglandin E2 receptors EP3 and EP4 on mouse and human β-cell survival and proliferation.
Carboneau BA, Allan JA, Townsend SE, Kimple ME, Breyer RM, Gannon M. Carboneau BA, et al. Mol Metab. 2017 Apr 5;6(6):548-559. doi: 10.1016/j.molmet.2017.04.002. eCollection 2017 Jun. Mol Metab. 2017. PMID: 28580285 Free PMC article. - Third isoform of the prostaglandin-E-receptor EP3 subtype with different C-terminal tail coupling to both stimulation and inhibition of adenylate cyclase.
Irie A, Sugimoto Y, Namba T, Harazono A, Honda A, Watabe A, Negishi M, Narumiya S, Ichikawa A. Irie A, et al. Eur J Biochem. 1993 Oct 1;217(1):313-8. doi: 10.1111/j.1432-1033.1993.tb18248.x. Eur J Biochem. 1993. PMID: 8223569 - [Cooperation of two subtypes of PGE2 receptor, Gi coupled EP3 and Gs coupled EP2 or EP4 subtype].
Hatae N. Hatae N. Yakugaku Zasshi. 2003 Oct;123(10):837-43. doi: 10.1248/yakushi.123.837. Yakugaku Zasshi. 2003. PMID: 14577329 Review. Japanese. - Functional domains essential for Gs activity in prostaglandin EP2 and EP3 receptors.
Sugimoto Y, Nakato T, Kita A, Hatae N, Tabata H, Tanaka S, Ichikawa A. Sugimoto Y, et al. Life Sci. 2003 Dec 5;74(2-3):135-41. doi: 10.1016/j.lfs.2003.09.002. Life Sci. 2003. PMID: 14607240
Cited by
- Prostaglandin E receptors as targets for ischemic stroke: Novel evidence and molecular mechanisms of efficacy.
Li L, Sluter MN, Yu Y, Jiang J. Li L, et al. Pharmacol Res. 2021 Jan;163:105238. doi: 10.1016/j.phrs.2020.105238. Epub 2020 Oct 11. Pharmacol Res. 2021. PMID: 33053444 Free PMC article. Review. - International Union of Basic and Clinical Pharmacology. CIX. Differences and Similarities between Human and Rodent Prostaglandin E2 Receptors (EP1-4) and Prostacyclin Receptor (IP): Specific Roles in Pathophysiologic Conditions.
Norel X, Sugimoto Y, Ozen G, Abdelazeem H, Amgoud Y, Bouhadoun A, Bassiouni W, Goepp M, Mani S, Manikpurage HD, Senbel A, Longrois D, Heinemann A, Yao C, Clapp LH. Norel X, et al. Pharmacol Rev. 2020 Oct;72(4):910-968. doi: 10.1124/pr.120.019331. Pharmacol Rev. 2020. PMID: 32962984 Free PMC article. - Prostanoid receptor antagonists: development strategies and therapeutic applications.
Jones RL, Giembycz MA, Woodward DF. Jones RL, et al. Br J Pharmacol. 2009 Sep;158(1):104-45. doi: 10.1111/j.1476-5381.2009.00317.x. Epub 2009 Jul 15. Br J Pharmacol. 2009. PMID: 19624532 Free PMC article. Review. - Small molecules targeting cyclooxygenase/prostanoid cascade in experimental brain ischemia: Do they translate?
Jiang J, Yu Y. Jiang J, et al. Med Res Rev. 2021 Mar;41(2):828-857. doi: 10.1002/med.21744. Epub 2020 Oct 22. Med Res Rev. 2021. PMID: 33094540 Free PMC article. Review. - EP3 (prostaglandin E2 receptor 3) expression is a prognostic factor for progression-free and overall survival in sporadic breast cancer.
Semmlinger A, von Schoenfeldt V, Wolf V, Meuter A, Kolben TM, Kolben T, Zeder-Goess C, Weis F, Gallwas J, Wuerstlein R, Hermelink K, Schmoeckel E, Harbeck N, Mayr D, Mahner S, Jeschke U, Ditsch N. Semmlinger A, et al. BMC Cancer. 2018 Apr 16;18(1):431. doi: 10.1186/s12885-018-4286-9. BMC Cancer. 2018. PMID: 29661238 Free PMC article.
References
- Jemal, A., Siegel, R., Ward, E., Murray, T., Xu, J., and Thun, M. J. (2007) CA Cancer J. Clin. 57 43-66 - PubMed
- Eberhart, C. E., Coffey, R. J., Radhika, A., Giardiello, F. M., Ferrenbach, S., and DuBois, R. N. (1994) Gastroenterology 107 1183-1188 - PubMed
- Kargman, S. L., O'Neill, G. P., Vickers, P. J., Evans, J. F., Mancini, J. A., and Jothy, S. (1995) Cancer Res. 55 2556-2559 - PubMed
- Sano, H., Kawahito, Y., Wilder, R. L., Hashiramoto, A., Mukai, S., Asai, K., Kimura, S., Kato, H., Kondo, M., and Hla, T. (1995) Cancer Res. 55 3785-3789 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources