Rho GDP dissociation inhibitor-mediated disruption of Rho GTPase activity impairs lens fiber cell migration, elongation and survival - PubMed (original) (raw)
Rho GDP dissociation inhibitor-mediated disruption of Rho GTPase activity impairs lens fiber cell migration, elongation and survival
Rupalatha Maddala et al. Dev Biol. 2008.
Abstract
To explore the role of the Rho GTPases in lens morphogenesis, we overexpressed bovine Rho GDP dissociation inhibitor (Rho GDI alpha), which serves as a negative regulator of Rho, Rac and Cdc42 GTPase activity, in a lens-specific manner in transgenic mice. This was achieved using a chimeric promoter of delta-crystallin enhancer and alpha A-crystallin, which is active at embryonic day 12. Several individual transgenic (Tg) lines were obtained, and exhibited ocular specific phenotype comprised of microphthalmic eyes with lens opacity. The overexpression of bovine Rho GDI alpha disrupted membrane translocation of Rho, Rac and Cdc42 GTPases in Tg lenses. Transgenic lenses also revealed abnormalities in the migration pattern, elongation and organization of lens fibers. These changes appeared to be associated with impaired organization of the actin cytoskeleton and cell-cell adhesions. At E14.5, the size of the Rho GDI alpha Tg lenses was larger compared to wild type (WT) and the central lens epithelium and differentiating fibers exhibited an abnormal increase of bromo-deoxy-uridine incorporation. Postnatal Tg eyes, however, were much smaller in size compared to WT eyes, revealing increased apoptosis in the disrupted lens fibers. Taken together, these data demonstrate a critical role for Rho GTPase-dependent signaling pathways in processes underlying morphogenesis, fiber cell migration, elongation and survival in the developing lens.
Figures
Fig.1
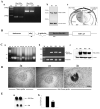
Constitutive expression and distribution of RhoGDIα in the mouse lens, design of bovine RhoGDIα transgene construct, transgene expression, and ocular phenotype. A. Constitutive expression and distribution of RhoGDIα in P1 and P7 mouse lenses. a. RT-PCR analysis of RhoGDIα and RhoGDIγ expression in P1 and P7 mouse lens; b. Immunoblot analysis of RhoGDIα in P1 and P7 mouse lens and c. Immunohistochemical localization of RhoGDIα in P1 mouse lens. The right half of the panel c depicts the background staining detected using secondary antibody alone B. Schematic diagram of transgenic vector showing insertion of a bovine RhoGDIα coding sequence under the chimeric promoter that contains the mouse αA-crystallin promoter (αAp) linked to the chick δ1-crystallin lens enhancer (δ-enh). A polyadenylation signal sequence from the human growth hormone gene (hGH pA), and rabbit β-globulin intron sequences were added at the 3’ and 5’ends of the RhoGDIα cDNA, respectively. Locations of the primers used for genotying by PCR are shown with arrows. C. Bovine RhoGDIα transgene insertion, expression and distribution in the transgenic mice. a. PCR screening of transgenic and nontransgenic mice using tail DNA, b. Confirmation of bovine RhoGDIα expression in the transgenic mouse lens by RT-PCR analysis, and c. Immunoblotting analysis using anti-RhoGDIα monoclonal antibody to confirm increased levels of total RhoGDIα (both transgenic and constitutive) D. In situ hybridization analysis of bovine RhoGDIα transgene expression in the transgenic mice and note that expression is localized specifically to the lens tissue. E. Transgenic eyes derived from one month-old mice were much smaller in size compared to WT littermates (a) and the wet weights of the Tg eyes were significantly reduced (>50% with P<0.01) compared to those from WT mice (b).
Fig. 2
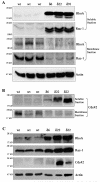
Defective membrane translocation, and elevated levels of Rho, Rac and Cdc42 GTPases in RhoGDIα transgenic mouse lenses. Distribution of Rho and Rac GTPases (Panel A), and Cdc42 (panel B) in the soluble and membrane rich fractions of lens tissue obtained from P1 Tg and WT mice. To evaluate the influence of RhoGDIα overexpression on the membrane localization of Rho, Rac and Cdc42 GTPases in the Tg and WT lenses, soluble and membrane protein fractions were isolated from the P1 lenses (pooled samples) and Rho, Rac and Cdc42 protein levels were evaluated by immunoblot analysis using equal amounts of protein. These analyses were carried out in multiple and a representative immunoblot is shown here. The soluble fractions were also immunoblotted for actin to confirm equal protein loading among the different samples. C. Increased levels of Rho, Rac and Cdc42 GTPases in transgenic lens homogenates (800xg supernatant). Lens homogenates (from pooled lenses) derived from the Tg and WT mice were subjected to immunoblot analysis. The same samples used in panel B and C were also immunoblotted for actin to confirm equal protein loading. The Tg line 91 was not included in these analyses.
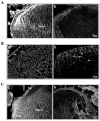
Fig. 3. Abnormal lens phenotype in the embryonic RhoGDIα transgenic mice
A. Hematoxylin and Eosin stained sagittal sections of E14.5 WT and RhoGDIα Tg eyes. The E14.5 Tg lenses from all three lines exhibit a marginal increase in size compared to wild type lenses. The magnification of WT and Tg lenses is the same. E14.5 lenses from Tg lines δ22 and δ91 show abnormal fiber cell morphology and defective secondary fiber cell organization. B. Higher magnification of E14.5 Tg lenses (white asterisks in panel A figures indicate the magnified area shown in panel B) at the equatorial region reveals defective fiber cell orientation and migration (indicated with arrows).
Fig. 4. Abnormal lens phenotype in the neonatal RhoGDIα transgenic mice
A. Hematoxylin and Eosin stained sagittal sections of P1 WT and RhoGDIα Tg eyes. P1 Tg lenses from all three lines reveal gross morphological changes especially in the lens fibers including defective lens fiber cell attachment with lens epithelium, accumulation of vacuoles (arrow heads), blunted fiber cell anterior tips (arrows) and ruptured posterior capsule. B. Abnormal morphology of lens epithelium in Tg lenses with multilayered nuclei and thickened epithelium (arrow heads). Globulization of secondary lens fibers and detachment of primary lens fiber cell anterior tips from the apical surface of lens epithelial cells in the transgenic mice (indicated with arrows). C. While in WT, the posterior lens fibers migrate towards the suture line, in the Tg lenses the fibers are completely disorganized, displaying an abnormal morphology and accumulate of nuclei. White asterisks in panel A figures indicate the magnified area shown in panel B and C.
Fig. 4. Abnormal lens phenotype in the neonatal RhoGDIα transgenic mice
A. Hematoxylin and Eosin stained sagittal sections of P1 WT and RhoGDIα Tg eyes. P1 Tg lenses from all three lines reveal gross morphological changes especially in the lens fibers including defective lens fiber cell attachment with lens epithelium, accumulation of vacuoles (arrow heads), blunted fiber cell anterior tips (arrows) and ruptured posterior capsule. B. Abnormal morphology of lens epithelium in Tg lenses with multilayered nuclei and thickened epithelium (arrow heads). Globulization of secondary lens fibers and detachment of primary lens fiber cell anterior tips from the apical surface of lens epithelial cells in the transgenic mice (indicated with arrows). C. While in WT, the posterior lens fibers migrate towards the suture line, in the Tg lenses the fibers are completely disorganized, displaying an abnormal morphology and accumulate of nuclei. White asterisks in panel A figures indicate the magnified area shown in panel B and C.
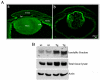
Fig. 5. Abnormal BrdU incorporation in RhoGDIα transgenic lenses
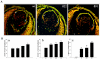
A. In vivo BrdU incorporation was performed to evaluate lens epithelial cell proliferation status in Tg mice. In WT E15.5 lenses, the BrdU-labeling was localized (bright yellow fluorescence) to the central and equatorial regions of the epithelium (anterior region between the two white arrows). However, compared to WT lenses, in the Tg lenses (δ6, δ22 and δ91), the entire central epithelium revealed intense BrdU positive labeling (bright yellow fluorescence). Additionally, most of the nuclei in the fiber cells were positive for BrdU incorporation in the Tg lenses. Lens sections were co-labeled with propidium iodide to localize the cell nucleus (red fluorescence). Representative images from the Tg line δ22 and δ91 are shown in this figure. B. Quantitative differences in BrdU incorporation in the lens central epithelium (panel b) and differentiating fibers (panel c) between WT and Tg mice. Compared to WT lenses, % BrdU incorporation was found to be significantly higher in the central epithelium (panel b) and fibers (panel c) in transgenic lenses. Panel a, indicates changes in the total number of nuclei in central epithelium between WT and Tg lenses. * P<0.05, ** P>0.01; 6-8 lens sections were analyzed from two independent mice. Since the various WT lenses showed identical values, data derived from WT littermate of Tg line 22 was given as a representative (Fig. 5A, B).
Fig. 6
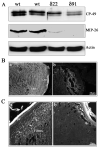
Defective organization of water channel and gap junctional proteins in the RhoGDIα transgenic lens fibers. A. P1 Tg lens homogenates (800xg supernatants) showed decreased levels of fiber-specific beaded filament protein (CP-49, also called phakanin), water channel protein Aquaporin-0 (also called MIP-26) compared to WT lenses, as determined by western blot analysis. Actin, which was immunoblotted to confirm equality of protein loading, indicated no differences between the WT and Tg specimens. B. Immunofluorescence distribution of aquaporin-0 in P1 WT and Tg lens cryosections. While immunolabelling for aquaporin-0 shows a fiber cell specific distribution along the cell membrane in WT lenses, a much weaker immunostaining that was not specifically localized to the lens fiber cell membrane, was noted in the case of Tg lenses. C. Immunofluorescence distribution of connexin-50, a lens fiber-specific gap junction protein in Tg and WT lenses. Similar to aquaporin-0, WT lenses reveal a specific and punctuate immunopositive staining pattern for connexin-50 distributed along the fiber cell membrane (panel a, indicated with arrows), while there was no detectable immunopositive labeling for connexin-50 in the Tg lenses (b). The lens capsule exhibits a non-specific bright staining in both WT and Tg specimens.
Fig. 7. Increased apoptotic cell death of the lens fibers in the RhoGDIα transgenic eyes
P1 WT and Tg lens cryosections were examined for apoptotic cell death by detecting the digoxigenin-nucleotides using a fluorescein-tagged anti-digoxigenin antibody. These same lens sections were also co-stained with propidium iodide to localize nuclei. While WT lens sections (A) revealed no TUNEL positive cells in the fiber cells, degenerating lens fibers of Tg lens showed increased TUNEL positive immunostaining in the central (B) and posterior (C) regions (indicated with arrows). Epithelium from both WT and Tg lenses showed sporadic TUNEL positive cells but with no significant difference between the two types of lenses. A representative image is shown based on multiple analyses.
Fig. 8
Defective organization of actin filament and adherens junction-associated β-catenin in the RhoGDIα transgenic lenses. Sagittal (A) and equatorial (B) plane cryosections from P1 WT and Tg lenses were stained for filamentous actin with rhodamin-phalloidin, and fluorescence staining images were captured with a confocal microscope. While the actin filament network is distributed uniformly in the WT lens epithelium and fibers along the cell membrane in the tissue sections derived from the sagittal plane (A; panel a), in the Tg lens fibers (A; panel b), actin distribution is distorted with irregular organization and a much reduced staining. Images viewed from the equatorial plane of the WT lens sections (B; panel a) revealed a uniform and clustered localization of actin filament at the angles of the short side of the hexagonal fiber cells (indicated with arrows), in the lens epithelial cells, it is distributed from basal to apical side along the cell membrane. On the other hand, in the Tg lenses (B; panel b), the fiber cells exhibit an asymmetric and distorted cell morphology, and actin filaments do not exhibit the atypical clustered localization at the angles of the hexagonal fiber cells, being distributed uniformly along the fiber cell membrane (indicated with arrows). C. Distribution of β-catenin in P1 WT and Tg lenses. The sagittal P1 lens cryosections immunostained with anti-β-catenin polyclonal antibody in conjunction with FITC-conjugated secondary antibody showed distribution of β-catenin at the intercellular junctions of the epithelial cells, and in fibers it is distributed along the fiber cell membrane, spreading from anterior to posterior tips (panel a). In the epithelium of Tg lenses (panel b), β-catenin distribution was found to be similar to that of WT lenses, localizing to the intercellular junctions (arrows). In the fibers, however, its distribution was found to be distorted and did not show uniform localization along the fiber cell membrane and exhibited a much weaker staining compared to WT lenses.
Fig. 9. Increased αB-crystallin phosphorylation in the RhoGDIα transgenic lens epithelium
A. P1 WT and Tg lens cryosections immunostainined with a Ser-59 phosphospecific αB-crystallin antibody revealed the presence of phosphorylated αB-crystallin in the epithelium and fibers cells (a). However, while distribution of phosphorylated αB-crystallin was uniform between the epithelium and fiber cells of WT lenses, the Tg lenses (b), exhibited a very intense staining for phosphorylated αB-crystallin throughout the epithelium, including at the equatorial and central regions (arrows). A representative photograph is presented in this figure based on multiple analyses using lens sections derived from the different Tg lines. B. Transgenic lens total (800x supernatants) or insoluble fractions (100,000xg pellets) immunoblotted with phosphospecific αB-crystallin antibody showed a significant increase in the levels of phosphorylated αB-crystallin, both in total homogenate and membrane fractions compared to the WT lenses. Actin was probed in the same samples to confirm loading equivalence for protein.
Similar articles
- Abundant expression of ponsin, a focal adhesion protein, in lens and downregulation of its expression by impaired cytoskeletal signaling.
Rao PV, Maddala R. Rao PV, et al. Invest Ophthalmol Vis Sci. 2009 Apr;50(4):1769-77. doi: 10.1167/iovs.08-2909. Epub 2008 Nov 21. Invest Ophthalmol Vis Sci. 2009. PMID: 19029030 Free PMC article. - Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse.
Maddala R, Deng PF, Costello JM, Wawrousek EF, Zigler JS, Rao VP. Maddala R, et al. Lab Invest. 2004 Jun;84(6):679-92. doi: 10.1038/labinvest.3700105. Lab Invest. 2004. PMID: 15094715 - GDIs: central regulatory molecules in Rho GTPase activation.
DerMardirossian C, Bokoch GM. DerMardirossian C, et al. Trends Cell Biol. 2005 Jul;15(7):356-63. doi: 10.1016/j.tcb.2005.05.001. Trends Cell Biol. 2005. PMID: 15921909 Review. - Ras-related GTPases and the cytoskeleton.
Hall A. Hall A. Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
Cited by
- Modulation of N-cadherin junctions and their role as epicenters of differentiation-specific actin regulation in the developing lens.
Leonard M, Zhang L, Zhai N, Cader A, Chan Y, Nowak RB, Fowler VM, Menko AS. Leonard M, et al. Dev Biol. 2011 Jan 15;349(2):363-77. doi: 10.1016/j.ydbio.2010.10.009. Epub 2010 Oct 20. Dev Biol. 2011. PMID: 20969840 Free PMC article. - Aquaporin-0 interacts with the FERM domain of ezrin/radixin/moesin proteins in the ocular lens.
Wang Z, Schey KL. Wang Z, et al. Invest Ophthalmol Vis Sci. 2011 Jul 7;52(8):5079-87. doi: 10.1167/iovs.10-6998. Invest Ophthalmol Vis Sci. 2011. PMID: 21642618 Free PMC article. - Understanding the role of growth factors in embryonic development: insights from the lens.
Lovicu FJ, McAvoy JW, de Iongh RU. Lovicu FJ, et al. Philos Trans R Soc Lond B Biol Sci. 2011 Apr 27;366(1568):1204-18. doi: 10.1098/rstb.2010.0339. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21402581 Free PMC article. Review. - Integrin-linked kinase deletion in the developing lens leads to capsule rupture, impaired fiber migration and non-apoptotic epithelial cell death.
Cammas L, Wolfe J, Choi SY, Dedhar S, Beggs HE. Cammas L, et al. Invest Ophthalmol Vis Sci. 2012 May 17;53(6):3067-81. doi: 10.1167/iovs.11-9128. Invest Ophthalmol Vis Sci. 2012. PMID: 22491404 Free PMC article. - A role for epha2 in cell migration and refractive organization of the ocular lens.
Shi Y, De Maria A, Bennett T, Shiels A, Bassnett S. Shi Y, et al. Invest Ophthalmol Vis Sci. 2012 Feb 1;53(2):551-9. doi: 10.1167/iovs.11-8568. Invest Ophthalmol Vis Sci. 2012. PMID: 22167091 Free PMC article.
References
- Bassnett S, Missey H, Vucemilo I. Molecular architecture of the lens fiber cell basal membrane complex. J Cell Sci. 1999;112(Pt 13):2155–65. - PubMed
- Beebe DC, Vasiliev O, Guo J, Shui YB, Bassnett S. Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Invest Ophthalmol Vis Sci. 2001;42:727–34. - PubMed
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. - PubMed
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY12201/EY/NEI NIH HHS/United States
- R24 EY014795/EY/NEI NIH HHS/United States
- R01 EY013573/EY/NEI NIH HHS/United States
- P30EY005722/EY/NEI NIH HHS/United States
- EY013573/EY/NEI NIH HHS/United States
- R01 EY013146/EY/NEI NIH HHS/United States
- R01 EY012201/EY/NEI NIH HHS/United States
- EY14795/EY/NEI NIH HHS/United States
- P30 EY005722/EY/NEI NIH HHS/United States
- EY13146/EY/NEI NIH HHS/United States
- R01 EY012201-09/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous