Functions of interneurons in mouse cerebellum - PubMed (original) (raw)
Comparative Study
Functions of interneurons in mouse cerebellum
Neal H Barmack et al. J Neurosci. 2008.
Abstract
The output signal of Purkinje cells is conveyed by the modulated discharge of simple spikes (SSs) often ascribed to mossy fiber-granule cell-parallel fiber inputs to Purkinje cell dendrites. Although generally accepted, this view lacks experimental support. We can address this view by controlling afferent signals that reach the cerebellum over climbing and mossy fiber pathways. Vestibular primary afferents constitute the largest mossy fiber projection to the uvula-nodulus. The discharge of vestibular primary afferent mossy fibers increases during ipsilateral roll tilt. The discharge of SSs decreases during ipsilateral roll tilt. Climbing fiber discharge [complex spikes (CSs)] increases during ipsilateral roll tilt. These observations suggest that the modulation of SSs during vestibular stimulation cannot be attributed directly to vestibular mossy fiber afferents. Rather we suggest that interneurons driven by vestibular climbing fibers may determine SS modulation. We recorded from cerebellar interneurons (granule, unipolar brush, Golgi, stellate, basket, and Lugaro cells) and Purkinje cells in the uvula-nodulus of anesthetized mice during vestibular stimulation. We identified all neuronal types by juxtacellular labeling with neurobiotin. Granule, unipolar brush, stellate, and basket cells discharge in phase with ipsilateral roll tilt and in phase with CSs. Golgi cells discharge out of phase with ipsilateral roll tilt and out of phase with CSs. The phases of stellate and basket cell discharge suggests that their activity could account for the antiphasic behavior of CSs and SSs. Because Golgi cells discharge in phase with SSs, Golgi cell activity cannot account for SS modulation. The sagittal array of Golgi cell axon terminals suggests that they contribute to the organization of discrete parasagittal vestibular zones.
Figures
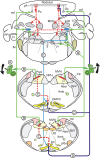
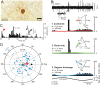
Figure 1.
Vestibular-cerebellar circuitry. Solid lines indicate excitatory pathways. Dashed lines indicate inhibitory pathways. A, Roll tilt onto the left side increases vestibular primary afferent discharge. B, Vestibular primary afferents project to ipsilateral parasolitary nucleus (Psol), Y-group (Y), and granule cell layer of nodulus. C, Psol projects to ipsilateral β-nucleus and DMCC. D, Climbing fibers from the left β-nucleus and DMCC project to contralateral (right) nodulus. E, Y-group projects to contralateral dorsal cap (DC), β-nucleus, and DMCC. Amb, Nucleus ambiguus; cf, climbing fiber; DVN, descending vestibular nucleus; LVN, lateral vestibular nucleus; MVN medial vestibular nucleus; SVN, superior vestibular nucleus; Fl, flocculus; Gc, granule cell; icp, inferior cerebellar peduncle; IntP, interpositus nucleus; LCN, lateral cerebellar nucleus; MCN, medial cerebellar nucleus; LRN, lateral reticular nucleus; Nsol, nucleus solitarius; PFl, paraflocculus; Pc, Purkinje cell; pf, parallel fiber; Psol, parasolitary nucleus; SpV, spinal trigeminal nucleus; VI, abducens nucleus; X, dorsal motor nucleus of the vagus; XII, hypoglossal nerve; 8n, vestibular nerve.
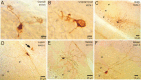
Figure 2.
Six interneurons identified by juxtacellular stimulation. Interneurons filled with neurobiotin by juxtacellular stimulation are shown. A, Granule cell. B, Unipolar brush cell. C, Golgi cell. D, Lugaro cell. The arrow indicates four granule cells that were colabeled after juxtacellular staining of the Lugaro cell. E, Stellate cell. F, Basket cell. Note that the scale bar differs for each cell type. gl, Granule cell layer; ml, molecular layer; Pl, Purkinje cell layer.
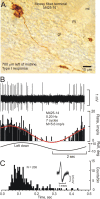
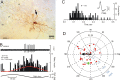
Figure 3.
Purkinje cell CSs respond in phase with ipsilateral sinusoidal roll tilt. A, Juxtacellularly labeled Purkinje cell M428-7 was located 800 μm to the right of the midline in folia 10. The arrow indicates a molecular layer interneuron that was colabeled after juxtacellular staining of the Purkinje cell. B, CSs and SSs were modulated by sinusoidal roll tilt. Peristimulus histogram shows that the discharge of CSs (black bars) increased and SSs (gray bars) decreased during ipsilateral roll tilt. The histograms were fitted with cosine functions (CSs, red line; SSs, green line). C, An ISIH for M428-7 shows a broad distribution of intervals for both CSs and SSs. Note the different time scales for CSs and SSs. The CS is illustrated with five and the SS is illustrated with 25 superimposed traces. D, A polar plot of the 269/318 responsive Purkinje cells illustrates antiphasic discharge of CSs (red circles) and SSs (open green circles). The population vector for CSs (black arrow) had M = 0.25 imp/s and ϕ = 43°. For the population vector for SSs, M = 1.3 imp/s and ϕ = 209°. Responses for M428-7 are represented by the filled black (CS) and yellow (SS) circles. For abbreviations, see Figure 2.
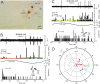
Figure 4.
Mossy fiber terminal type I response. A, Juxtacellularly labeled mossy fiber terminal M425-14 was located 700 μm to the left of the midline in folium 9c. B, It had a type I response. Its discharge increased during ipsilateral roll tilt. The peristimulus histogram (black bars) was fitted with a cosine function (red line). C, An ISIH shows a broad distribution of intervals. The action potential is illustrated with 25 superimposed traces. For abbreviations, see Figure 2.
Figure 5.
Granule cells respond to roll tilt with low rates and with bursts. A, Granule cell M399-3 was located 50 μm to the right of the midline in folium 9c. B, Vestibularly modulated slow responses were interspersed in 9 of 11 stimulus cycles with high-frequency bursts. Three peristimulus histograms were plotted and included different combinations of bursts and regular discharge: bursts and regular discharge were fitted with a cosine function (red line; 1); bursts only were plotted (cosine function, green line; 2); and regular discharges, without bursts, were plotted (cosine function, blue line; 3). The vectors in B1–B3 indicate the average M and ϕ for each condition. C, An ISIH shows two discrete peaks of interspike intervals. The first (red arrowhead) corresponds to the high-frequency bursts. The second consists of more broadly distributed ISIs and corresponds to regular discharge. The action potential is illustrated with 25 superimposed traces. D, A polar plot for the population of 28 vestibularly responsive granule cells yielded a vector (red arrow) with M = 0.7 imp/s and ϕ = 5°. The combined response of M399-3 is indicated by the filled black circle.
Figure 6.
UBCs respond to roll tilt. A, Juxtacellularly labeled UBC M272-6 was located 350 μm to the left of the midline in folium 10. B, The activity of M272-6 increased during ipsilateral roll tilt. The peristimulus histogram was fitted with a cosine function (blue line). C, An ISIH shows a narrow peak of interspike intervals. The action potential is illustrated with 25 superimposed traces. D, A polar plot for the population of 27 vestibularly responsive UBCs yielded a vector (black arrow) with M = 5.4 imp/s and ϕ = 32°. The response of M272-6 is indicated by the filled yellow circle.
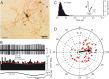
Figure 7.
Golgi cell activity increases during contralateral roll tilt. A, Juxtacellularly labeled Golgi cell M338-2 was located 300 μm to the left of the midline in folium 10. The arrow indicates a granule cell that was colabeled by juxtacellular staining of the Golgi cell. B, Golgi cell M338-2 discharged in phase with contralateral roll tilt velocity. Its peristimulus histogram was fitted with a cosine function (red line). C, An ISIH for M338-2 shows a broad distribution of intervals. The action potential is illustrated with 10 superimposed traces. D, A polar plot for the population of 18 vestibularly responsive Golgi cells yields a vector (black arrow) with M = 0.7 imp/s and ϕ = 180°. The response of Golgi cell M338-2 is indicated by the filled green circle. For abbreviations, see Figure 2.
Figure 8.
Parasagittal organization of Golgi cells. Nine juxtacellularly labeled Golgi cells are drawn in the sagittal plane through folia 8–10. Each is characterized by profusely branching, fine, varicose axons within the granule cell layer. The distance of each cell from the midline is listed. Colors are used to help distinguish the processes of different Golgi cells. For abbreviations, see Figure 2.
Figure 9.
Stellate cells respond to ipsilateral roll tilt. A, Juxtacellularly labeled stellate cell M415-3 was located 250 μm left of the midline in folium 9c. B, The activity of M415–3 was modulated by ipsilateral roll tilt. The peristimulus histogram was fitted with a cosine function (red line). C, An ISIH shows a relatively broad peak of interspike intervals. The action potential is illustrated with 25 superimposed traces. D, A polar plot for the population of 37 vestibularly responsive stellate cells yields a vector (black arrow) with M = 2.0 imp/s and ϕ = 11°. The response of M415-3 is indicated by the filled green circle. For abbreviations, see Figure 2.
Figure 10.
Basket cells respond to ipsilateral roll tilt. A, Photomicrograph shows two separately labeled cells; a basket cell (M372-4) and a granule cell (M372-3) located in folium 10, 100 μm to the right of the midline. Discharges of both cells were modulated by ipsilateral roll tilt. B, C, Both cells were fitted with a cosine function (basket cell-red line, granule cell-yellow line). The third trace for each cell is an ISIH. Each shows a relatively broad distribution of interspike intervals. Action potentials are illustrated with 25 superimposed traces. The granule cell lacked the bursts that characterized the discharge of other granule cells. D, A polar plot for the population of four of five vestibularly responsive basket cells yields a vector (red arrow) with M = 0.8 imp/s and ϕ = 1°. The response of basket cell M372-4 is indicated by the filled green circle. The response of granule cell M372-3, not included in the computation of the basket cell vector, is indicated by the filled blue circle. For abbreviations, see Figure 2.
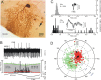
Figure 11.
Topography and polarity of interneuronal responses to roll tilt about longitudinal axis. The location of each neurobiotin-labeled interneuron is indicated on a two-dimensional map. A, Granule cells. B, Unipolar brush cells. C, Golgi cells. D, Stellate cells. The polarity of interneuronal responses to ipsilateral roll tilt about the longitudinal axis is classified as “in phase” (filled circles) or “out of phase” (open circles). Unresponsive cells are indicated by X. F, Table summarizes responses for all labeled interneurons. G, Sagittal section through the uvula-nodulus shows its correspondence with the two-dimensional representations in A–D.
Similar articles
- Climbing fibers mediate vestibular modulation of both "complex" and "simple spikes" in Purkinje cells.
Barmack NH, Yakhnitsa V. Barmack NH, et al. Cerebellum. 2015 Oct;14(5):597-612. doi: 10.1007/s12311-015-0725-1. Cerebellum. 2015. PMID: 26424151 Review. - Microlesions of the inferior olive reduce vestibular modulation of Purkinje cell complex and simple spikes in mouse cerebellum.
Barmack NH, Yakhnitsa V. Barmack NH, et al. J Neurosci. 2011 Jul 6;31(27):9824-35. doi: 10.1523/JNEUROSCI.1738-11.2011. J Neurosci. 2011. PMID: 21734274 Free PMC article. - Antiphasic Purkinje cell responses in mouse uvula-nodulus are sensitive to static roll-tilt and topographically organized.
Yakhnitsa V, Barmack NH. Yakhnitsa V, et al. Neuroscience. 2006 Dec 1;143(2):615-26. doi: 10.1016/j.neuroscience.2006.08.006. Epub 2006 Sep 14. Neuroscience. 2006. PMID: 16973298 - Cerebellar climbing fibers modulate simple spikes in Purkinje cells.
Barmack NH, Yakhnitsa V. Barmack NH, et al. J Neurosci. 2003 Aug 27;23(21):7904-16. doi: 10.1523/JNEUROSCI.23-21-07904.2003. J Neurosci. 2003. PMID: 12944521 Free PMC article. - Topsy turvy: functions of climbing and mossy fibers in the vestibulo-cerebellum.
Barmack NH, Yakhnitsa V. Barmack NH, et al. Neuroscientist. 2011 Apr;17(2):221-36. doi: 10.1177/1073858410380251. Epub 2011 Feb 28. Neuroscientist. 2011. PMID: 21362689 Free PMC article. Review.
Cited by
- Autonomous Purkinje cell activation instructs bidirectional motor learning through evoked dendritic calcium signaling.
Bonnan A, Rowan MMJ, Baker CA, Bolton MM, Christie JM. Bonnan A, et al. Nat Commun. 2021 Apr 12;12(1):2153. doi: 10.1038/s41467-021-22405-8. Nat Commun. 2021. PMID: 33846328 Free PMC article. - Graded Control of Climbing-Fiber-Mediated Plasticity and Learning by Inhibition in the Cerebellum.
Rowan MJM, Bonnan A, Zhang K, Amat SB, Kikuchi C, Taniguchi H, Augustine GJ, Christie JM. Rowan MJM, et al. Neuron. 2018 Sep 5;99(5):999-1015.e6. doi: 10.1016/j.neuron.2018.07.024. Epub 2018 Aug 16. Neuron. 2018. PMID: 30122378 Free PMC article. - Deploying and Optimizing Embodied Simulations of Large-Scale Spiking Neural Networks on HPC Infrastructure.
Feldotto B, Eppler JM, Jimenez-Romero C, Bignamini C, Gutierrez CE, Albanese U, Retamino E, Vorobev V, Zolfaghari V, Upton A, Sun Z, Yamaura H, Heidarinejad M, Klijn W, Morrison A, Cruz F, McMurtrie C, Knoll AC, Igarashi J, Yamazaki T, Doya K, Morin FO. Feldotto B, et al. Front Neuroinform. 2022 May 19;16:884180. doi: 10.3389/fninf.2022.884180. eCollection 2022. Front Neuroinform. 2022. PMID: 35662903 Free PMC article. - Modulated discharge of Purkinje and stellate cells persists after unilateral loss of vestibular primary afferent mossy fibers in mice.
Barmack NH, Yakhnitsa V. Barmack NH, et al. J Neurophysiol. 2013 Nov;110(10):2257-74. doi: 10.1152/jn.00352.2013. Epub 2013 Aug 21. J Neurophysiol. 2013. PMID: 23966673 Free PMC article. - Dynamic metabotropic control of intrinsic firing in cerebellar unipolar brush cells.
Russo MJ, Yau HJ, Nunzi MG, Mugnaini E, Martina M. Russo MJ, et al. J Neurophysiol. 2008 Dec;100(6):3351-60. doi: 10.1152/jn.90533.2008. Epub 2008 Oct 22. J Neurophysiol. 2008. PMID: 18945818 Free PMC article.
References
- Alley K, Baker R, Simpson JI. Afferents to the vestibulo-cerebellum and the origin of the visual climbing fibers in the rabbit. Brain Res. 1975;98:582–589. - PubMed
- Andersen P, Eccles JC, Voorhoeve PE. Postsynaptic inhibition of cerebellar Purkinje cells. J Neurophysiol. 1964;27:1138–1153. - PubMed
- Barmack NH, Shojaku H. Vestibular and visual signals evoked in the uvula-nodulus of the rabbit cerebellum by natural stimulation. J Neurophysiol. 1995;74:2573–2589. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources