Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules - PubMed (original) (raw)
Comparative Study
Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules
Xue-Jun Li et al. Stem Cells. 2008 Apr.
Abstract
Specification of distinct cell types from human embryonic stem cells (hESCs) is key to the potential application of these naïve pluripotent cells in regenerative medicine. Determination of the nontarget differentiated populations, which is lacking in the field, is also crucial. Here, we show an efficient differentiation of motor neurons ( approximately 50%) by a simple sequential application of retinoid acid and sonic hedgehog (SHH) in a chemically defined suspension culture. We also discovered that purmorphamine, a small molecule that activates the SHH pathway, could replace SHH for the generation of motor neurons. Immunocytochemical characterization indicated that cells differentiated from hESCs were nearly completely restricted to the ventral spinal progenitor fate (NKX2.2+, Irx3+, and Pax7-), with the exception of motor neurons (HB9+) and their progenitors (Olig2+). Thus, the directed neural differentiation system with small molecules, even without further purification, will facilitate basic and translational studies using human motoneurons at a minimal cost.
Conflict of interest statement
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
Figures
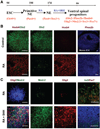
Figure 1. Near complete specification of ventral spinal progenitors from human ESCs in suspension culture
(A): Schematic procedure for ventral spinal progenitor differentiation.(B): Primitive NE (d10), after treatment with RA for 1 week, were isolated and cultured in suspension without (control; upper row) or with (lower row) RA for another week (total 24 d). RA induced the expressionof Hoxb4 but inhibited Otx2 expression.Very few cells expressed Phox2b in the RA-treated cultures. (C): Posteriorized neuralprogenitors (d17) were cultured in the absence(upper row) or presence (lower row)of SHH, and expression of transcriptional factors along the dorsal-ventral axis was examined at d28. In the absence of SHH, a small population of cells expressed Nkx2.2 and Olig2, whereas more cells were positive for Irx3, among which some also expressed Pax7. When SHH (100 ng/ml) was added (lower row), a large portion of cells expressedOlig2 or Nkx2.2, whereas few cells were positive for Irx3 and no cells werepositively stained for Pax7 (second row).Blue indicates Hoechst-stained nuclei. Scalebars = 50 µm. Abbreviations: d, days; E, embryonic day; NE, neuroepithelia; RA, retinoidacid; SHH, sonic hedgehog.
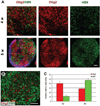
Figure 2. Highly efficient generation of motoneurons in the continual presence of sonic hedgehog
(A): Olig2+ motoneuron progenitors peaked at approximately 4 weeks after differentiation, when HB9+ postmitotic motoneurons began to appear. Subsequently, the population of HB9+ motoneurons increased and peaked at 5 weeks. (B): A confocal image showing the separation of most Olig2- and HB9-positive cells at 5 weeks after differentiation. (C): Diagram showing the change of population of Olig2+ and HB9+ cells at 4–5 weeks after differentiation. Data are presented as mean ± SEM; n = 15–17. Blue indicates Hoechst-stained nuclei. Scale bars = 50 µm. Abbreviation: w, weeks.
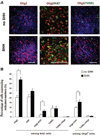
Figure 3. SHH promotes proliferation of Olig2+ progenitors
(A): Olig2-enriched clusters were dissociated and plated on polyornithine/laminin-coated coverslips in the neural medium supplemented with B27 in the absence or presence of SHH (100 ng/ml) for 24 hours. More Olig2+ and Ki67+/ Olig2+ cells were seen with SHH than without SHH. TUNEL staining showed no difference between the SHH and non-SHH groups. Blue indicates Hoechst-stained nuclei. Scale bars = 50 µm. (B): Quantitative analyses indicated that there were more Olig2+, Ki67+/Olig2+ cells in the SHH-treated cultures than in the control cultures without SHH, whereas the numbers of Ki67+ and TUNEL+ cells in the total differentiated cells were similar between the SHH and non-SHH-treated groups. Data are presented as mean ± SEM; n = 7–8.*, analysis of variance test between SHH and non-SHH-treated groups, p < .05. Abbreviations: SHH, sonic hedgehog; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling.
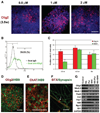
Figure 4. Efficient generation of spinal progenitors and motor neurons by purmorphamine
(A): Caudalized neuroepithelia (NE) (day 17) were treated with RA and different concentrations of purmorphamine. At 3.5 weeks after human ESC differentiation, Olig2 was induced by purmorphamine in a dose-dependent manner. (B): Cell populations were quantified by fluorescence-activated cell sorting, as exemplified by Olig2-expressing cells in the purmorphamine (1 µM) group. (C): Diagram showing time-dependent change of population of Olig2+ and HB9+ cells after differentiation. Data are presented as mean ± SEM; n = 5–7. (D): At 4.5 weeks, the expression of Olig2 and HB9 increased to more than 40%. (E): After another week of differentiation in adherent cultures, most HB9+ motoneurons also expressed ChAT. (F): Synapsin-positive neurites colocalized with α-BTX-stained acetylcholine receptors on the surface of the myotube (arrow) after 2 weeks of coculture of motor neurons and C2C12 myoblasts, as shown on a 0.5-µm confocal section. (G): Reverse transcription-polymerase chain reaction analyses indicated expression of transcriptional factors by caudalized NE that were cultured with RA (0.1 µM), purmorphamine, SHH, or purmorphamine plus RA for 1 week (day 24). Blue indicates Hoechst-stained nuclei. Scale bars = 50 µm (A, D, E) and 30 µm (F). Abbreviations: ChAT, choline acetyltransferase; Pur, purmorphamine; RA, retinoid acid; SHH, sonic hedgehog; w, weeks.
Similar articles
- Directed differentiation of neural-stem cells and subtype-specific neurons from hESCs.
Hu BY, Zhang SC. Hu BY, et al. Methods Mol Biol. 2010;636:123-37. doi: 10.1007/978-1-60761-691-7_8. Methods Mol Biol. 2010. PMID: 20336520 Free PMC article. - Notch signaling regulates motor neuron differentiation of human embryonic stem cells.
Ben-Shushan E, Feldman E, Reubinoff BE. Ben-Shushan E, et al. Stem Cells. 2015 Feb;33(2):403-15. doi: 10.1002/stem.1873. Stem Cells. 2015. PMID: 25335858 - Efficient derivation of NPCs, spinal motor neurons and midbrain dopaminergic neurons from hESCs at 3% oxygen.
Stacpoole SR, Bilican B, Webber DJ, Luzhynskaya A, He XL, Compston A, Karadottir R, Franklin RJ, Chandran S. Stacpoole SR, et al. Nat Protoc. 2011 Jul 28;6(8):1229-40. doi: 10.1038/nprot.2011.380. Nat Protoc. 2011. PMID: 21799491 Free PMC article. - Easy and rapid differentiation of embryonic stem cells into functional motoneurons using sonic hedgehog-producing cells.
Soundararajan P, Lindsey BW, Leopold C, Rafuse VF. Soundararajan P, et al. Stem Cells. 2007 Jul;25(7):1697-706. doi: 10.1634/stemcells.2006-0654. Epub 2007 Mar 29. Stem Cells. 2007. PMID: 17395777 - Retinoic Acid-Mediated Regulation of GLI3 Enables Efficient Motoneuron Derivation from Human ESCs in the Absence of Extrinsic SHH Activation.
Calder EL, Tchieu J, Steinbeck JA, Tu E, Keros S, Ying SW, Jaiswal MK, Cornacchia D, Goldstein PA, Tabar V, Studer L. Calder EL, et al. J Neurosci. 2015 Aug 19;35(33):11462-81. doi: 10.1523/JNEUROSCI.3046-14.2015. J Neurosci. 2015. PMID: 26290227 Free PMC article.
Cited by
- Directed Differentiation of V3 Interneurons from Mouse Embryonic Stem Cells.
Xu H, Sakiyama-Elbert SE. Xu H, et al. Stem Cells Dev. 2015 Nov 15;24(22):2723-32. doi: 10.1089/scd.2015.0122. Epub 2015 Aug 12. Stem Cells Dev. 2015. PMID: 26165862 Free PMC article. - Adult human neural stem cell therapeutics: Current developmental status and prospect.
Nam H, Lee KH, Nam DH, Joo KM. Nam H, et al. World J Stem Cells. 2015 Jan 26;7(1):126-36. doi: 10.4252/wjsc.v7.i1.126. World J Stem Cells. 2015. PMID: 25621112 Free PMC article. Review. - Plastin 3 is upregulated in iPSC-derived motoneurons from asymptomatic SMN1-deleted individuals.
Heesen L, Peitz M, Torres-Benito L, Hölker I, Hupperich K, Dobrindt K, Jungverdorben J, Ritzenhofen S, Weykopf B, Eckert D, Hosseini-Barkooie SM, Storbeck M, Fusaki N, Lonigro R, Heller R, Kye MJ, Brüstle O, Wirth B. Heesen L, et al. Cell Mol Life Sci. 2016 May;73(10):2089-104. doi: 10.1007/s00018-015-2084-y. Epub 2015 Nov 16. Cell Mol Life Sci. 2016. PMID: 26573968 Free PMC article. - Differentiation of neural precursors and dopaminergic neurons from human embryonic stem cells.
Zhang XQ, Zhang SC. Zhang XQ, et al. Methods Mol Biol. 2010;584:355-66. doi: 10.1007/978-1-60761-369-5_19. Methods Mol Biol. 2010. PMID: 19907987 Free PMC article. - Differentiation of human oligodendrocytes from pluripotent stem cells.
Hu BY, Du ZW, Zhang SC. Hu BY, et al. Nat Protoc. 2009;4(11):1614-22. doi: 10.1038/nprot.2009.186. Epub 2009 Oct 15. Nat Protoc. 2009. PMID: 19834476 Free PMC article.
References
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
- Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. - PubMed
- Barberi T, Bradbury M, Dincer Z, et al. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 HD003352/HD/NICHD NIH HHS/United States
- R21 NS055261/NS/NINDS NIH HHS/United States
- R21-NS055261/NS/NINDS NIH HHS/United States
- R01-NS045926/NS/NINDS NIH HHS/United States
- R01 NS045926/NS/NINDS NIH HHS/United States
- P01 NS057778/NS/NINDS NIH HHS/United States
- P30 HD03352/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources