ORAI and store-operated calcium influx in human airway smooth muscle cells - PubMed (original) (raw)
ORAI and store-operated calcium influx in human airway smooth muscle cells
Samantha E Peel et al. Am J Respir Cell Mol Biol. 2008 Jun.
Abstract
The initial bronchoconstrictor response of the asthmatic airway depends on airway smooth muscle (ASM) contraction. Intracellular calcium is a key signaling molecule, mediating a number of responses, including proliferation, gene expression, and contraction of ASM. Ca(2+) influx through receptor-operated calcium (ROC) or store-operated calcium (SOC) channels is believed to mediate longer term signals. The mechanisms of SOC activation in ASM remain to be elucidated. Recent literature has identified the STIM and ORAI proteins as key signaling players in the activation of the SOC subtype; calcium release-activated channel current (I(CRAC)) in a number of inflammatory cell types. However, the role for these proteins in activation of SOC in smooth muscle is unclear. We have previously demonstrated a role for STIM1 in SOC channel activation in human ASM. The aim of this study was to investigate the expression and define the potential roles of the ORAI proteins in SOC-associated Ca(2+) influx in human ASM cells. Here we show that knockdown of ORAI1 by siRNA resulted in reduced thapsigargin- or cyclopiazonic acid (CPA)-induced Ca(2+) influx, without affecting Ca(2+) release from stores or basal levels. CPA-induced inward currents were also reduced in the ORAI1 knockdown cells. We propose that ORAI1 together with STIM1 are important contributors to SOC entry in ASM cells. These data extend the major tissue types in which these proteins appear to be major determinants of SOC influx, and suggest that modulation of these pathways may prove useful in the treatment of bronchoconstriction.
Figures
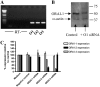
**Figure 1.
Expression of ORAI homologs in human airway smooth muscle (HASM) and siRNA-targeted knockdown of ORAI homologs. (A) mRNA expression of ORAI1, 2, and 3 in HASM cells using reverse transcriptase–polymerase chain reaction (RT-PCR) (O1: ORAI1, O2: ORAI2, O3: ORAI3). PCR products were sequenced to confirm expression. No products were detected in the RT samples (i.e., RNA samples that have not been reverse transcribed) to confirm no genomic DNA contamination. (B) Western blot analysis of ORAI1 protein expression after ORAI1 siRNA transfection; expression of smooth muscle α-actin was assessed as a control for equal protein loading. (C) siRNA-mediated knockdown of ORAI1, 2, and 3 mRNA assessed by real-time, quantitative PCR; the effects of individual siRNAs on expression of all ORAI homologs were assessed. The level of ORAI mRNA expression in HASM cells transfected with siRNA was compared relative to an untreated control, which was set to 100%. Results are expressed as the mean ± SEM, cDNA samples were tested in triplicate and the graph represents data from three separate transfections.
**Figure 2.
Effects of ORAI1, 2, or 3 mRNA knockdown on cyclopiazonic acid (CPA)– and thapsigargin (TG)-induced Ca2+ influx. (Ai and Aii) Representative raw traces illustrating the CPA-induced changes in [Ca2+]i (presented as fluorescence intensity [FI]) in HASM cells transfected with ORAI1 siRNA (Ai) and ORAI3 siRNA (Aii), compared with HASM cells transfected with a negative control siRNA. CPA (10 μM) was added to the cells in the presence of low extracellular Ca2+ (0.1 mM) followed by the restoration of 2 mM Ca2+ as indicated. (Aiii) Summary of the data illustrated in (Ai) and (Aii) showing averaged changes in fluorescence after 2 mM Ca2+ restoration (averaged data from 8 separate experiments, at least 6 repeats within each experiment). (Bi and Bii) Representative raw traces illustrating the TG-induced changes in [Ca2+]i in HASM cells transfected with ORAI1 siRNA (Bi) and ORAI3 siRNA (Bii), compared with control cells. TG (1 μM) was added to the cells in the presence of low extracellular Ca2+ (0.1 mM) for 20 minutes before assay, followed by the restoration of 2 mM Ca2+ as indicated. (Biii) Summary of the data illustrated in (Bi) and (Bii) showing averaged changes in fluorescence after 2 mM Ca2+ restoration (averaged data from 4 separate experiments, at least 6 repeats within each experiment). Results are expressed as % changes ± SEM compared with control. Data are indicated as statistically significant with *P < 0.05 and **P < 0.01.
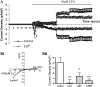
**Figure 3.
Effects of ORAI1 knockdown on CPA-induced inward currents in single HASM cells assessed by whole cell patch clamp. (A) Time course of current density (inward currents measured at −80 mV, outward current measured at +80 mV); each point represents mean data ± SEM of all cells in each group; control cells treated with negative control siRNA (n = 12), ORAI1 suppressed cells (n = 17). (Bi) Representative current–voltage (I-V) relationships at point 1 from the time course. (Bii) Bar chart illustrating CPA-sensitive inward current density at point 1 (measured at −80 mV) in cells transfected with negative control or ORAI1 siRNA. Data are indicated as statistically significant with *P < 0.05.
**Figure 4.
Inhibitory effects of 100 μM La3+, 100 μM Gd3+, and 50 μM 2-aminoethoxydiphenylborane (2-APB) on CPA-induced inward currents in single HASM cells assessed by whole cell patch clamp. (A) Time course of current density (inward currents measured at −80 mV, outward current measured at +80 mV) showing control cells versus cells treated with 100 μM La3+, each point represents mean data ± SEM of all cells in each group; control cells (n = 9), La3+-treated cells (n = 9). (Bi) Representative current–voltage (I-V) relationships at point 1 from the time course. (Bii) A bar chart illustrating CPA-sensitive inward current density at point 1 (measured at −80 mV) of control cells compared with cells treated with 100 μM La3+, 100 μM Gd3+, and 50 μM 2-APB. Data are indicated as statistically significant with *P < 0.05, **P < 0.01.
Similar articles
- STIM1 regulates platelet-derived growth factor-induced migration and Ca2+ influx in human airway smooth muscle cells.
Suganuma N, Ito S, Aso H, Kondo M, Sato M, Sokabe M, Hasegawa Y. Suganuma N, et al. PLoS One. 2012;7(9):e45056. doi: 10.1371/journal.pone.0045056. Epub 2012 Sep 11. PLoS One. 2012. PMID: 22984609 Free PMC article. - A key role for STIM1 in store operated calcium channel activation in airway smooth muscle.
Peel SE, Liu B, Hall IP. Peel SE, et al. Respir Res. 2006 Sep 20;7(1):119. doi: 10.1186/1465-9921-7-119. Respir Res. 2006. PMID: 16987424 Free PMC article. - Intricate interaction between store-operated calcium entry and calcium-activated chloride channels in pulmonary artery smooth muscle cells.
Forrest AS, Angermann JE, Raghunathan R, Lachendro C, Greenwood IA, Leblanc N. Forrest AS, et al. Adv Exp Med Biol. 2010;661:31-55. doi: 10.1007/978-1-60761-500-2_3. Adv Exp Med Biol. 2010. PMID: 20204722 - Store-Independent Orai Channels Regulated by STIM.
Zhang X, Gueguinou M, Trebak M. Zhang X, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review. - Orai channel-mediated Ca2+ signals in vascular and airway smooth muscle.
Spinelli AM, Trebak M. Spinelli AM, et al. Am J Physiol Cell Physiol. 2016 Mar 15;310(6):C402-13. doi: 10.1152/ajpcell.00355.2015. Epub 2015 Dec 30. Am J Physiol Cell Physiol. 2016. PMID: 26718630 Free PMC article. Review.
Cited by
- Role of TRPC1 channel in skeletal muscle function.
Zanou N, Shapovalov G, Louis M, Tajeddine N, Gallo C, Van Schoor M, Anguish I, Cao ML, Schakman O, Dietrich A, Lebacq J, Ruegg U, Roulet E, Birnbaumer L, Gailly P. Zanou N, et al. Am J Physiol Cell Physiol. 2010 Jan;298(1):C149-62. doi: 10.1152/ajpcell.00241.2009. Epub 2009 Oct 21. Am J Physiol Cell Physiol. 2010. PMID: 19846750 Free PMC article. - Store-operated CRAC channels: function in health and disease.
Parekh AB. Parekh AB. Nat Rev Drug Discov. 2010 May;9(5):399-410. doi: 10.1038/nrd3136. Epub 2010 Apr 16. Nat Rev Drug Discov. 2010. PMID: 20395953 Review. - Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration.
Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd'heuil D, Trebak M. Bisaillon JM, et al. Am J Physiol Cell Physiol. 2010 May;298(5):C993-1005. doi: 10.1152/ajpcell.00325.2009. Epub 2010 Jan 27. Am J Physiol Cell Physiol. 2010. PMID: 20107038 Free PMC article. - Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1.
Lyfenko AD, Dirksen RT. Lyfenko AD, et al. J Physiol. 2008 Oct 15;586(20):4815-24. doi: 10.1113/jphysiol.2008.160481. Epub 2008 Sep 4. J Physiol. 2008. PMID: 18772199 Free PMC article. - Pharmacologic modulation of experimentally induced allergic asthma.
Fraňová S, Strapková A, Mokrý J, Sutovská M, Jošková M, Sadloňová V, Antošová M, Pavelčíková D, Flešková D, Nosáĺová G. Fraňová S, et al. Interdiscip Toxicol. 2011 Mar;4(1):27-32. doi: 10.2478/v10102-011-0006-x. Interdiscip Toxicol. 2011. PMID: 21577281 Free PMC article.
References
- Hall IP. Second messengers, ion channels and pharmacology of airway smooth muscle. Eur Respir J 2000;15:1120–1127. - PubMed
- Sweeney M, McDaniel SS, Platoshyn O, Zhang S, Yu Y, Lapp BR, Zhao Y, Thistlethwaite PA, Yuan JX. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol 2002;92:1594–1602. - PubMed
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 2006;441:179–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous