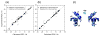Affinity makes the difference: nonselective interaction of the UBA domain of Ubiquilin-1 with monomeric ubiquitin and polyubiquitin chains - PubMed (original) (raw)
Affinity makes the difference: nonselective interaction of the UBA domain of Ubiquilin-1 with monomeric ubiquitin and polyubiquitin chains
Daoning Zhang et al. J Mol Biol. 2008.
Abstract
Ubiquilin/PLIC proteins belong to the family of UBL-UBA proteins implicated in the regulation of the ubiquitin-dependent proteasomal degradation of cellular proteins. A human presenilin-interacting protein, ubiquilin-1, has been suggested as potential therapeutic target for treating Huntington's disease. Ubiquilin's interactions with mono- and polyubiquitins are mediated by its UBA domain, which is one of the tightest ubiquitin binders among known ubiquitin-binding domains. Here we report the three-dimensional structure of the UBA domain of ubiquilin-1 (UQ1-UBA) free in solution and in complex with ubiquitin. UQ1-UBA forms a compact three-helix bundle structurally similar to other known UBAs, and binds to the hydrophobic patch on ubiquitin with a K(d) of 20 microM. To gain structural insights into UQ1-UBA's interactions with polyubiquitin chains, we have mapped the binding interface between UQ1-UBA and Lys48- and Lys63-linked di-ubiquitins and characterized the strength of UQ1-UBA binding to these chains. Our NMR data show that UQ1-UBA interacts with the individual ubiquitin units in both chains in a mode similar to its interaction with mono-ubiquitin, although with an improved binding affinity for the chains. Our results indicate that, in contrast to UBA2 of hHR23A that has strong binding preference for Lys48-linked chains, UQ1-UBA shows little or no binding selectivity toward a particular chain linkage or between the two ubiquitin moieties in the same chain. The structural data obtained in this study provide insights into the possible structural reasons for the diversity of polyubiquitin chain recognition by UBA domains.
Figures
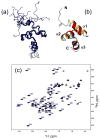
Figure 1
NMR-derived structures of the Ubiquilin1 UBA domain. (a) The ensemble of 10 solution NMR structures of UQ1-UBA, and (b) a ribbon representation of one of the structures. (c) The 1H-15N HSQC spectrum of UQ1-UBA domain. All molecular drawings in this paper were made using MolMol .
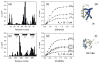
Figure 2
Chemical shift perturbation mapping of the interaction between UQ1-UBA and Ub. Amide CSPs in (a) monoUb and (b) UQ1-UBA at the endpoint of titration as a function of residue number. Panels (c) and (d) show representative titration curves for the perturbed residues in Ub and UQ1-UBA, respectively. These data depict the CSPs as a function of the molar ratio of the titrant and the 15N-labeled protein under the observation. The curves were obtained by fitting the CSPs to a 1:1 binding model (Methods). Panels (e) and (f) show a ribbon representation of the 3D structures of Ub and UQ1-UBA, with the residues exhibiting strong CSPs (Δδ >0.15ppm) shown in the CPK mode and colored blue and light blue, respectively. Note that the Δδ values for K48 (>1 ppm) and Q49 (~0.7 ppm) exceed the vertical scale used in panel (a).
Figure 3
Paramagnetic relaxation enhancement effect in the UQ1-UBA/Ub complex induced by the spin label. The top and bottom rows correspond to SL attached to Ub sites C48 and C12, respectively. (a, b) and (c, d) depict experimental PREs (circles) observed in SL-Ub and UQ1-UBA, respectively. The solid lines represent PREs back-calculated for the fitted SL position. Panels (e) and (f) show cartoon representations of the relative orientation of Ub and UQ1-UBA (similar to that in the calculated structure) with the reconstructed position of the unpaired electron of the SL shown as a sphere and the residues with significant PREs marked with thick backbone. The PRE-affected residues and the reconstructed spheres derived from measurements on SL-Ub are colored blue, those from UQ1-UBA measurements (in the presence of SL-Ub) are colored light blue. The side chains of K48 and T12 (mutated to a Cys for spin labeling) are shown in red sticks, as indicated. The reconstruction placed the unpaired electron of the SL at a distance of 7.2 Å from the Cα atom of K48 and at 8.8 Å from Cα of T12, the corresponding distances from Cβ atoms were 6.2 Å and 7.8 Å, respectively. Flexible unstructured C-terminal residues 72-76 in Ub and the 10 N-terminal residues in UQ1-UBA were excluded from the fits shown here; including these residues in the analysis had little effect on the SL positions. Also shown (panel (g)) is the chemical structure of the spin label used in this study.
Figure 4
The structure of the monoUb:UQ1-UBA complex. (a) The final ensemble of 10 lowest-energy NMR structures and a cartoon representation of the representative structure of the complex. (b) A close-up view of some intermolecular contacts at the Ub:UQ1-UBA binding interface. (c) Two possible relative orientations of Ub and UQ1-UBA agree with the RDC data. These conformations differ by a 180° rotation of one of the domains (in this case, UQ1-UBA) about the Z-axis of the alignment tensor; the grey rods indicate the principal axes of the tensor. Spheres represent the positions of the spin labels “reported” by the individual domains, determined from C48-spin labeling data (Fig. 3e). These structures were obtained from those in panel (a) by moving the two proteins away from each other along the Z-axis and rotating the complex around this axis by ~30°. (d) Same structures as in (c) (rotated around the Z axis) with the spin labels reconstructed from C12-SL experiments (Fig. 3f). The coloring of the SL-representing spheres in (c) and (d) is the same as in Fig. 3, i.e. the SL positions derived from measurements on SL-Ub and UQ1-UBA are colored blue and light blue, respectively. The pairs of residues that give intermolecular NOEs are shown in ball-and-stick in panels (c, d) and colored green (Q49 - L584) and orange (G47 - N561). Throughout this figure, Ub is colored blue and UQ1-UBA is light blue.
Figure 5
NMR characterization of the changes in the overall tumbling and backbone dynamics of UQ1-UBA and monoUb upon complex formation. Shown are the 15N relaxation rates (_R_1, _R_2) and hetero-NOE for both binding partners, UQ1-UBA (left panels) and Ub (right panels), in the free (open circles) and bound (solid circles) states.
Figure 6
Comparison of the experimental and back-calculated RDCs derived from the UQ1-UBA structures indicates structural changes in UQ1-UBA upon Ub binding. The RDC data for the (a) free and (b) Ub-bound UQ1-UBA were fit to UQ1-UBA structures in the free and Ub-bound states. The solid line corresponds to absolute agreement. (c) Superimposition of the UQ1-UBA structures in the free (light blue) and Ub-bound (blue) states.
Figure 7
Chemical shift perturbation mapping of Ub2’s surface involved in UQ1-UBA binding. Shown are CSPs as a function of residue number in (a) distal and (b) proximal domains in K48-Ub2, (c) distal and (d) proximal domains in K63-Ub2, and (e, f) ribbon representations of the corresponding Ub2s colored by the strength of the CSPs. Note that the Δδ values for K48 (>1 ppm) and Q49 (~0.7 ppm) exceed the vertical scale used here. The location of G76 (distal Ub) and the side chain (shown in red stick) of K48 or K63 (proximal Ub) that form the isopeptide bond linking the two Ubs in Ub2 is indicated in (e) and (f).
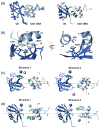
Figure 8
Comparison of the structure of (a) UQ1-UBA/monoUb complex derived here with the published structures of monoUb complexes with (b) Dsk2-UBA, (c) Ede1-UBA, and (d) Cue2-CUE1 domains (PDB codes 1WR1, 2G3Q, and 1OTR, respectively). Helices in UBAs are colored green (α1), khaki (α2), and magenta (α3) to guide the eye.
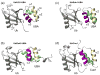
Figure 9
Structural models show how UQ1-UBA can bind (a) K63- and (b) K48-linked Ub2 chains and help rationalize (c,d) the differences in linkage selectivity between UQ1-UBA and hHR23A-UBA2. The structures in (a, b) were obtained by superimposition of the UQ1-UBA/Ub complex onto the distal and proximal Ubs of each chain, i.e. assuming that UQ1-UBA interacts with each Ub unit in the same way as with monoUb. The latter is justified by the fact that the CSPs in each Ub in these chains upon UQ1-UBA binding are almost identical to those in monoUb (cf Figs 2a and 7). The K63-Ub2 structure is from ref. , the K48-Ub2 structure shown in (b) corresponds to a fully open conformation of the chain reported in refs. ; . Comparison of the intermolecular contacts stabilizing the hHR23A-UBA2/K48-Ub2 complex (panel (c)) with those in a hypothetical model of a similar complex for UQ1-UBA (panel (d)) shows that the interactions that favor the formation of a 1:1 complex in the former are missing in the latter. The structure model in panel (d) was obtained by replacing the distal-Ub/UBA2 pair in (c) with the Ub/UQ1-UBA structure determined in this study (Fig. 8a); this replacement is justified by the fact that the CSPs observed both in the distal Ub and in UQ1-UBA are essentially the same as in the monoUb/UQ1-UBA complex. In both structures (c,d), the “canonical” Ub-binding side (loop 1 and helix α3) of the corresponding UBA domain is in contact with the hydrophobic patch on the distal Ub, and only the side chains of residues forming contacts between the UBA and the proximal Ub or the Ub-Ub linker are shown in ball-and-stick, colored green (UBA) and cyan (Ub). In all these Ub2 structures (grey) the distal Ub is on the left, the proximal Ub is on the right. The location of G76 (distal Ub) and the side chain (shown in red stick) of K48 or K63 (proximal Ub) that form the isopeptide bond linking the two Ubs in Ub2 is indicated. The UBAs bound to the distal and proximal Ubs are colored green and blue, respectively.
Similar articles
- Structural analysis of the UBA domain of X-linked inhibitor of apoptosis protein reveals different surfaces for ubiquitin-binding and self-association.
Tse MK, Hui SK, Yang Y, Yin ST, Hu HY, Zou B, Wong BC, Sze KH. Tse MK, et al. PLoS One. 2011;6(12):e28511. doi: 10.1371/journal.pone.0028511. Epub 2011 Dec 15. PLoS One. 2011. PMID: 22194841 Free PMC article. - Structural determinants for selective recognition of a Lys48-linked polyubiquitin chain by a UBA domain.
Varadan R, Assfalg M, Raasi S, Pickart C, Fushman D. Varadan R, et al. Mol Cell. 2005 Jun 10;18(6):687-98. doi: 10.1016/j.molcel.2005.05.013. Mol Cell. 2005. PMID: 15949443 - Rad23 ubiquitin-associated domains (UBA) inhibit 26 S proteasome-catalyzed proteolysis by sequestering lysine 48-linked polyubiquitin chains.
Raasi S, Pickart CM. Raasi S, et al. J Biol Chem. 2003 Mar 14;278(11):8951-9. doi: 10.1074/jbc.m212841200. J Biol Chem. 2003. PMID: 12643283 - Structural and functional studies of mutations affecting the UBA domain of SQSTM1 (p62) which cause Paget's disease of bone.
Layfield R, Ciani B, Ralston SH, Hocking LJ, Sheppard PW, Searle MS, Cavey JR. Layfield R, et al. Biochem Soc Trans. 2004 Nov;32(Pt 5):728-30. doi: 10.1042/BST0320728. Biochem Soc Trans. 2004. PMID: 15493999 Review. - Ubiquitin-binding domains.
Hurley JH, Lee S, Prag G. Hurley JH, et al. Biochem J. 2006 Nov 1;399(3):361-72. doi: 10.1042/BJ20061138. Biochem J. 2006. PMID: 17034365 Free PMC article. Review.
Cited by
- Novel TDP2-ubiquitin interactions and their importance for the repair of topoisomerase II-mediated DNA damage.
Rao T, Gao R, Takada S, Al Abo M, Chen X, Walters KJ, Pommier Y, Aihara H. Rao T, et al. Nucleic Acids Res. 2016 Dec 1;44(21):10201-10215. doi: 10.1093/nar/gkw719. Epub 2016 Aug 19. Nucleic Acids Res. 2016. PMID: 27543075 Free PMC article. - Unbiased screen reveals ubiquilin-1 and -2 highly associated with huntingtin inclusions.
Rutherford NJ, Lewis J, Clippinger AK, Thomas MA, Adamson J, Cruz PE, Cannon A, Xu G, Golde TE, Shaw G, Borchelt DR, Giasson BI. Rutherford NJ, et al. Brain Res. 2013 Aug 2;1524:62-73. doi: 10.1016/j.brainres.2013.06.006. Epub 2013 Jun 15. Brain Res. 2013. PMID: 23774650 Free PMC article. - Structure and energetics of pairwise interactions between proteasome subunits RPN2, RPN13, and ubiquitin clarify a substrate recruitment mechanism.
VanderLinden RT, Hemmis CW, Yao T, Robinson H, Hill CP. VanderLinden RT, et al. J Biol Chem. 2017 Jun 9;292(23):9493-9504. doi: 10.1074/jbc.M117.785287. Epub 2017 Apr 25. J Biol Chem. 2017. PMID: 28442575 Free PMC article. - UBQLN2 restrains the domesticated retrotransposon PEG10 to maintain neuronal health in ALS.
Black HH, Hanson JL, Roberts JE, Leslie SN, Campodonico W, Ebmeier CC, Holling GA, Tay JW, Matthews AM, Ung E, Lau CI, Whiteley AM. Black HH, et al. Elife. 2023 Mar 23;12:e79452. doi: 10.7554/eLife.79452. Elife. 2023. PMID: 36951542 Free PMC article. - Two ZnF-UBP domains in isopeptidase T (USP5).
Avvakumov GV, Walker JR, Xue S, Allali-Hassani A, Asinas A, Nair UB, Fang X, Zuo X, Wang YX, Wilkinson KD, Dhe-Paganon S. Avvakumov GV, et al. Biochemistry. 2012 Feb 14;51(6):1188-98. doi: 10.1021/bi200854q. Epub 2012 Feb 3. Biochemistry. 2012. PMID: 22283393 Free PMC article.
References
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
- Di Fiore PP, Polo S, Hofmann K. When ubiquitin meets ubiquitin receptors: a signalling connection. Nat Rev Mol Cell Biol. 2003;4:491–7. - PubMed
- Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–6. - PubMed
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases