Brain morphometry with multiecho MPRAGE - PubMed (original) (raw)
Brain morphometry with multiecho MPRAGE
André J W van der Kouwe et al. Neuroimage. 2008.
Abstract
In brain morphometry studies using magnetic resonance imaging, several scans with a range of contrasts are often collected. The images may be locally distorted due to imperfect shimming in regions where magnetic susceptibility changes rapidly, and all scans may not be distorted in the same way. In multispectral studies it is critical that the edges of structures align precisely across all contrasts. The MPRAGE (MPR) sequence has excellent contrast properties for cortical segmentation, while multiecho FLASH (MEF) provides better contrast for segmentation of subcortical structures. Here, a multiecho version of the MPRAGE (MEMPR) is evaluated using SIENA and FreeSurfer. The higher bandwidth of the MEMPR results in reduced distortions that match those of the MEF while the SNR is recovered by combining the echoes. Accurate automatic identification of cortex and thickness estimation is frustrated by the presence of dura adjacent to regions such as the entorhinal cortex. In the typical MPRAGE protocol, dura and cortex are approximately isointense. However, dura has substantially smaller T2* than cortex. This information is represented in the multiple echoes of the MEMPR. An algorithm is described for correcting cortical thickness using T2*. It is shown that with MEMPR, SIENA generates more reliable percentage brain volume changes and FreeSurfer generates more reliable cortical models. The regions where cortical thickness is affected by dura are shown. MEMPR did not substantially improve subcortical segmentations. Since acquisition time is the same for MEMPR as for MPRAGE, and it has better distortion properties and additional T2* information, MEMPR is recommended for morphometry studies.
Figures
Figure 1
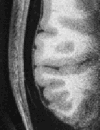
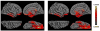
Partial axial slice through right side of brain showing cortex and adjacent isotense dura (0.35 mm isotropic MPRAGE).
Figure 2
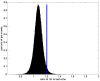
Histogram of ratio image values sampled 0.5 mm to 1 mm out from the gray/white junction. The blue line indicates the μ+2σ threshold. Note the Gaussian shape of the distribution.
Figure 3
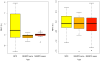
a. Comparison of percent brain volume change (PBVC) calculated by SIENA for 12 subjects on 2 scanners for MPR↑ vs. MPR↓, MEMPR↑↓ vs. MEMPR↓↑ and MEMPR↑↑ vs. MEMPR↓↓. b. Comparison of percent brain volume change (PBVC) calculated for 12 subjects using 6 sequence types for one Siemens 3 T TIM Trio vs. another. Note the scaling difference on the y axes.
Figure 4
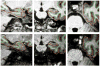
MPR↑ (top left), MPR↓ (bottom left), MEMPR↑↑ (top middle), MEMPR↓↓ (bottom middle), MEMPR↑↓ (top right) and MEMPR↓↑ (bottom right). All images show white matter surfaces (green) and the two pial surfaces (red) calculated from both the images with opposite readout directions (top vs. bottom rows).
Figure 5
Average displacement in mm between pial surfaces calculated from scans with opposite readout directions, displayed on right hemisphere rotated to show cortex where B0 offsets are greatest. MPR↑/↓ (left), MEMPR↑↑/↓↓ (middle) and MEMPR↑↓/↓↑ (right).
Figure 6
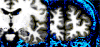
First (top) and fourth (bottom) echo of MEMPR showing difference in T2* contrast between dura and gray matter. In the first echo, as with single echo MPR, gray matter and dura are approximately isointense. All images show white matter surfaces (green) and the pial surface calculated without dura avoidance (yellow) and with dura avoidance (red).
Figure 7
Average cortical thickness differences in mm due to dura correction for MEMPR↑↑ (left) and MEMPR↑↓ (right).
Figure 8
Coronal slices through medial temporal susceptibility region (left) and medial frontal susceptibility region (right) showing regions where the first to fourth echo ratio image of the MEMPR exceeds the threshold for dura/non-cortex detection. The pial surface without dura avoidance is shown in yellow and the pial surface with dura avoidance is shown in red. Dura is avoided in the medial temporal susceptibility region, whereas the surface is largely unaffected in the medial frontal susceptibility area where dura does not interfere with cortex.
Figure 9
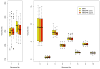
Volumes of brain structures averaged across subjects and scanners for three sequence types. 1=cerebral white matter, 2=cerebral cortex, 3=lateral ventricle, 4=inferior lateral ventricle, 5=thalamus proper, 6=caudate, 7=putamen, 8=pallidum, 9=hippocampus, 10=amygdala. Volumes are shown for left hemisphere – right hemisphere was similar.
Figure 10
Volume differences between brain structures scanned between the two scanners, averaged across subjects, for three sequence types. Structure numbers follow Figure 9 and are for the left hemisphere. Left and right hemisphere results were similar.
Figure 11
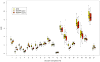
MPR and MEMPR (same, opposite direction readout) SNR values for linear discriminant between adjacent structure pairs. 1=white/gray, 2=white/thal., 3=white/caudate, 4=white/putamen, 5=white/pallidum, 6=white/hippo., 7=white/amygdala, 8=gray/hippo., 9=gray/amygdala, 10=putamen/pallidum, 11=hippo./gray, 12=hippo./amygdala, 13=hippo./lat. ventr., 14=hippo./inf. lat. ventr., 15=amygdala/white, 16=amygdala/gray, 17=amygdala/lat. ventr., 18=amygdala/inf.=lat. ventr., 19=thalamus/caudate, 20=thalamus/lat. ventr., 21=thalamus/pallidum.
Similar articles
- Brain morphometry reproducibility in multi-center 3T MRI studies: a comparison of cross-sectional and longitudinal segmentations.
Jovicich J, Marizzoni M, Sala-Llonch R, Bosch B, Bartrés-Faz D, Arnold J, Benninghoff J, Wiltfang J, Roccatagliata L, Nobili F, Hensch T, Tränkner A, Schönknecht P, Leroy M, Lopes R, Bordet R, Chanoine V, Ranjeva JP, Didic M, Gros-Dagnac H, Payoux P, Zoccatelli G, Alessandrini F, Beltramello A, Bargalló N, Blin O, Frisoni GB; PharmaCog Consortium. Jovicich J, et al. Neuroimage. 2013 Dec;83:472-84. doi: 10.1016/j.neuroimage.2013.05.007. Epub 2013 May 11. Neuroimage. 2013. PMID: 23668971 - SNR efficiency of combined bipolar gradient echoes: Comparison of three-dimensional FLASH, MPRAGE, and multiparameter mapping with VFA-FLASH and MP2RAGE.
Jutras JD, Wachowicz K, Gilbert G, De Zanche N. Jutras JD, et al. Magn Reson Med. 2017 Jun;77(6):2186-2202. doi: 10.1002/mrm.26306. Epub 2016 Jul 15. Magn Reson Med. 2017. PMID: 27416792 - Susceptibility-resistant variable-flip-angle turbo spin echo imaging for reliable estimation of cortical thickness: a feasibility study.
Lee H, Kim EY, Yang KS, Park J. Lee H, et al. Neuroimage. 2012 Jan 2;59(1):377-88. doi: 10.1016/j.neuroimage.2011.07.070. Epub 2011 Aug 5. Neuroimage. 2012. PMID: 21840400 - Measurement of Cortical Thickness and Volume of Subcortical Structures in Multiple Sclerosis: Agreement between 2D Spin-Echo and 3D MPRAGE T1-Weighted Images.
Vidal-Jordana A, Pareto D, Sastre-Garriga J, Auger C, Ciampi E, Montalban X, Rovira A. Vidal-Jordana A, et al. AJNR Am J Neuroradiol. 2017 Feb;38(2):250-256. doi: 10.3174/ajnr.A4999. Epub 2016 Nov 24. AJNR Am J Neuroradiol. 2017. PMID: 27884876 Free PMC article. - Multiple sclerosis lesions and irreversible brain tissue damage: a comparative ultrahigh-field strength magnetic resonance imaging study.
Sinnecker T, Mittelstaedt P, Dörr J, Pfueller CF, Harms L, Niendorf T, Paul F, Wuerfel J. Sinnecker T, et al. Arch Neurol. 2012 Jun;69(6):739-45. doi: 10.1001/archneurol.2011.2450. Arch Neurol. 2012. PMID: 22351849
Cited by
- Within-individual organization of the human cognitive cerebellum: Evidence for closely juxtaposed, functionally specialized regions.
Saadon-Grosman N, Du J, Kosakowski HL, Angeli PA, DiNicola LM, Eldaief MC, Buckner RL. Saadon-Grosman N, et al. Sci Adv. 2024 Nov 8;10(45):eadq4037. doi: 10.1126/sciadv.adq4037. Epub 2024 Nov 8. Sci Adv. 2024. PMID: 39514652 Free PMC article. - Study protocol: Cerebral autoregulation, brain perfusion, and neurocognitive outcomes after traumatic brain injury -CAPCOG-TBI.
Caldas J, Cardim D, Edmundson P, Morales J, Feng A, Ashley JD, Park C, Valadka A, Foreman M, Cullum M, Sharma K, Liu Y, Zhu D, Zhang R, Ding K. Caldas J, et al. Front Neurol. 2024 Oct 16;15:1465226. doi: 10.3389/fneur.2024.1465226. eCollection 2024. Front Neurol. 2024. PMID: 39479003 Free PMC article. - Enlarged perivascular space burden predicts declines in cognitive and functional performance.
Libecap TJ, Pappas CA, Bauer CE, Zachariou V, Raslau FD, Gold BT. Libecap TJ, et al. J Neurol Sci. 2024 Nov 15;466:123232. doi: 10.1016/j.jns.2024.123232. Epub 2024 Sep 12. J Neurol Sci. 2024. PMID: 39298972 - Blood-derived microRNAs are related to cognitive domains in the general population.
Melas K, Talevi V, Imtiaz MA, Etteldorf R, Estrada S, Krüger DM, Pena-Centeno T, Aziz NA, Fischer A, Breteler MMB. Melas K, et al. Alzheimers Dement. 2024 Oct;20(10):7138-7159. doi: 10.1002/alz.14197. Epub 2024 Aug 29. Alzheimers Dement. 2024. PMID: 39210637 Free PMC article. - Precision Network Modeling of Transcranial Magnetic Stimulation Across Individuals Suggests Therapeutic Targets and Potential for Improvement.
Sun W, Billot A, Du J, Wei X, Lemley RA, Daneshzand M, Nummenmaa A, Buckner RL, Eldaief MC. Sun W, et al. medRxiv [Preprint]. 2024 Sep 23:2024.08.15.24311994. doi: 10.1101/2024.08.15.24311994. medRxiv. 2024. PMID: 39185539 Free PMC article. Preprint.
References
- Mugler JP, 3rd, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magn Reson Med. 1990;15:152–157. - PubMed
- Mugler JP, 3rd, Brookeman JR. Rapid three-dimensional T1-weighted MR imaging with the MP-RAGE sequence. J Magn Reson Imaging. 1991;1:561–567. - PubMed
- Lichy MP, Wietek BM, Mugler JP, 3rd, Horger W, Menzel MI, Anastasiadis A, Siegmann K, Niemeyer T, Konigsrainer A, Kiefer B, Schick F, Claussen CD, Schlemmer HP. Magnetic resonance imaging of the body trunk using a single-slab, 3-dimensional, T2-weighted turbo-spin-echo sequence with high sampling efficiency (SPACE) for high spatial resolution imaging: initial clinical experiences. Invest Radiol. 2005;40:754–760. - PubMed
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. - PubMed
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. - PubMed
Publication types
MeSH terms
Grants and funding
- U24 RR021382/RR/NCRR NIH HHS/United States
- U24 RR021382-02/RR/NCRR NIH HHS/United States
- R01 RR016594-04/RR/NCRR NIH HHS/United States
- P41 RR014075-075753/RR/NCRR NIH HHS/United States
- P41-RR-14075/RR/NCRR NIH HHS/United States
- R21-EB02530/EB/NIBIB NIH HHS/United States
- R01 EB001550/EB/NIBIB NIH HHS/United States
- U54 EB005149-030006/EB/NIBIB NIH HHS/United States
- U54 EB005149-010019/EB/NIBIB NIH HHS/United States
- P41 RR014075-075752/RR/NCRR NIH HHS/United States
- R01-EB006758/EB/NIBIB NIH HHS/United States
- U24 RR021382-04/RR/NCRR NIH HHS/United States
- R01 NS052585-01A1/NS/NINDS NIH HHS/United States
- R01 EB006758/EB/NIBIB NIH HHS/United States
- U24 RR021382-01/RR/NCRR NIH HHS/United States
- U24 RR021382-037970/RR/NCRR NIH HHS/United States
- U54 EB005149-049001/EB/NIBIB NIH HHS/United States
- P41 RR014075-086766/RR/NCRR NIH HHS/United States
- BIRN002/PHS HHS/United States
- R01 RR016594-02/RR/NCRR NIH HHS/United States
- R01 EB001550-02/EB/NIBIB NIH HHS/United States
- U54 EB005149/EB/NIBIB NIH HHS/United States
- P41 RR014075-075751/RR/NCRR NIH HHS/United States
- R01 RR016594-03/RR/NCRR NIH HHS/United States
- U54-EB005149/EB/NIBIB NIH HHS/United States
- P41 RR014075/RR/NCRR NIH HHS/United States
- R01 NS052585/NS/NINDS NIH HHS/United States
- P41 RR014075-086767/RR/NCRR NIH HHS/United States
- R01 EB006758-01A1/EB/NIBIB NIH HHS/United States
- R01-EB001550/EB/NIBIB NIH HHS/United States
- U54 EB005149-029001/EB/NIBIB NIH HHS/United States
- R21 EB002530/EB/NIBIB NIH HHS/United States
- R01-NS052585-01/NS/NINDS NIH HHS/United States
- P41 RR014075-098602/RR/NCRR NIH HHS/United States
- R01 NS052585-02S1/NS/NINDS NIH HHS/United States
- R01 EB001550-01/EB/NIBIB NIH HHS/United States
- U54 EB005149-04S1/EB/NIBIB NIH HHS/United States
- U54 EB005149-03/EB/NIBIB NIH HHS/United States
- R01 NS052585-03/NS/NINDS NIH HHS/United States
- U24 RR021382-047976/RR/NCRR NIH HHS/United States
- P41 RR014075-098601/RR/NCRR NIH HHS/United States
- R01 EB001550-04/EB/NIBIB NIH HHS/United States
- R21 EB002530-02/EB/NIBIB NIH HHS/United States
- U54 EB005149-02/EB/NIBIB NIH HHS/United States
- R01-RR16594-01A1/RR/NCRR NIH HHS/United States
- U24-RR021382/RR/NCRR NIH HHS/United States
- U24 RR021382-037971/RR/NCRR NIH HHS/United States
- P41 RR014075-086765/RR/NCRR NIH HHS/United States
- R01 RR016594-01A1/RR/NCRR NIH HHS/United States
- R01 RR016594/RR/NCRR NIH HHS/United States
- U24 RR021382-047975/RR/NCRR NIH HHS/United States
- R01 NS052585-02/NS/NINDS NIH HHS/United States
- U54 EB005149-010006/EB/NIBIB NIH HHS/United States
- U54 EB005149-020006/EB/NIBIB NIH HHS/United States
- U54 EB005149-04/EB/NIBIB NIH HHS/United States
- U24 RR021382-03/RR/NCRR NIH HHS/United States
- R01 EB001550-03/EB/NIBIB NIH HHS/United States
- U54 EB005149-040006/EB/NIBIB NIH HHS/United States
- R21 EB002530-01/EB/NIBIB NIH HHS/United States
- U54 EB005149-01/EB/NIBIB NIH HHS/United States
- U54 EB005149-039001/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical