Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells - PubMed (original) (raw)
Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells
Zhonglin Xie et al. Circulation. 2008.
Abstract
Background: Metformin, one of most commonly used antidiabetes drugs, is reported to exert its therapeutic effects by activating AMP-activated protein kinase (AMPK); however, the mechanism by which metformin activates AMPK is poorly defined. The objective of the present study was to determine how metformin activates AMPK in endothelial cells.
Methods and results: Exposure of human umbilical vein endothelial cells or bovine aortic endothelial cells to metformin significantly increased AMPK activity and the phosphorylation of both AMPK at Thr172 and LKB1 at Ser428, an AMPK kinase, which was paralleled by increased activation of protein kinase C (PKC)-zeta, as evidenced by increased activity, phosphorylation (Thr410/403), and nuclear translocation of PKC-zeta. Consistently, either pharmacological or genetic inhibition of PKC-zeta ablated metformin-enhanced phosphorylation of both AMPK-Thr172 and LKB1-Ser428, suggesting that PKC-zeta might act as an upstream kinase for LKB1. Furthermore, adenoviral overexpression of LKB1 kinase-dead mutants abolished but LKB1 wild-type overexpression enhanced the effects of metformin on AMPK in bovine aortic endothelial cells. In addition, metformin increased the phosphorylation and nuclear export of LKB1 into the cytosols as well as the association of AMPK with LKB1 in bovine aortic endothelial cells. Similarly, overexpression of LKB1 wild-type but not LKB1 S428A mutants (serine replaced by alanine) restored the effects of metformin on AMPK in LKB1-deficient HeLa-S3 cells, suggesting that Ser428 phosphorylation of LKB1 is required for metformin-enhanced AMPK activation. Moreover, LKB1 S428A, like kinase-dead LKB1 D194A, abolished metformin-enhanced LKB1 translocation as well as the association of LKB1 with AMPK in HeLa-S3 cells. Finally, inhibition of PKC-zeta abolished metformin-enhanced coimmunoprecipitation of LKB1 with both AMPKalpha1 and AMPKalpha2.
Conclusions: We conclude that PKC-zeta phosphorylates LKB1 at Ser428, resulting in LKB1 nuclear export and hence AMPK activation.
Figures
Figure 1
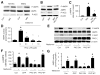
Inhibition of PKC-ζ attenuates metformin-enhanced AMPK activity. Confluent BAEC or HUVEC, with or without adenovirus infection, were preincubated with PKC-ζ_-PS for 30 minutes before being exposed to metformin. After treatment, the cells were lysaed and extracted. Both phosphorylated AMPK-Thr172 and ACC-Ser79 were detected in Western blots by using specific antibodies, as described in Methods. A, Metformin (Met) activates AMPK in BAEC and HUVEC. The blot is a representative of 6 blots obtained from 6 individual experiments. Con indicates control. B and C, Selective inhibition of PKC-ζ attenuated metformin-enhanced AMPK activity (n=6). ♣_P<0.05 (control [Con] vs metformin treated), †P<0.05 (metformin vs metformin plus PKC-ζ_-PS). D, PKC-ζ_-PS dose-dependently inhibits metformin-enhanced AMPK activity in BAEC (n=5). ♣_P<0.05 (control vs metformin), †_P<0.05 (metformin vs metformin plus PKC-ζ_-PS). E and F, Genetic inhibition of PKC-ζ with PKC-ζ_-DN, but not the adenovi-rus encoding GFP, attenuated metformin-enhanced phosphorylation of AMPK-Thr172 and ACC-Ser79 in BAEC. The blot is representative of 5 blots from 5 individual experiments (n=5). ♣_P<0.05 (compared with control), †_P<0.05 (metformin-treated vs metformin plus adenovirus). G, AMPK activity was assayed as described in Methods (n=4). ♣P<0.05 (compared with control), †P<0.05 (metformin-treated vs metformin plus adenovirus).
Figure 2
Metformin increases PKC-ζ phosphorylation and the translocation of PKC-ζ from cytosol to the membrane. Confluent BAEC were exposed to metformin (1 mmol/L, 1 hour), and the translocation of PKC-ζ and PKC-ζ phosphorylation was assayed as described in Methods. A, Metformin (Met) increased the phosphorylation of PKC-ζ in BAEC. The blot is a representative of 3 blots obtained from 3 independent experiments. Con indicates control. B, PKC-ζ activity was determined as described in Methods (n=4). ♣P<0.05 (compared with control [Con]), †P<0.05 (metformin-treated vs metformin plus adenovirus), #P<0.05 (PKC-ζ_-WT vs metformin plus WT adenovirus). C and D, Metformin increases the translocation of PKC-ζ to the membrane (n=5). ♣_P<0.05 (control vs metformin-treated). E and F, Metformin increases the translocation of PKC-ζ from cytosol into the nucleus. The blot is a representative of 3 blots from 3 individual experiments (n=5). ♣P<0.05 (control vs metformin-treated). G, Analysis of the purity of subcellular fractions. The subcellular fractions were prepared as described in Methods. Marker enzymes were detected by Western blot with the use of specific antibodies.
Figure 3
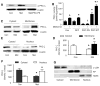
Neither PKA nor RSK is involved in metformin-enhanced AMPK activation in BAEC. A and B, Confluent BAEC were pretreated with protein kinase inhibitors for 30 minutes followed by treatment with metformin (1 mmol/L) for 1 hour, the cells were lysed, and phosphorylation of AMPK and LKB1 was detected by Western blot. The blot is a representative of blots from 3 different experiments. ♣P<0.05 compared with respective control (Con). C, BAEC were treated with metformin (Met) (1 mmol/L) for 1 hour, cell lysate was prepared, and the phosphorylation of PKC-ζ, PKAc, and RSK3 was detected by Western blot with the use of specific antibodies. The blot is a representative of 3 blots obtained from 3 individual experiments. D, LKB1 was immunoprecipitated, and PKC-ζ, PKAc, and RSK3 were detected by Western blotting. The blot is representative of blots from 3 different experiments. E, Confluent BAECs were transfected with PKC-_ζ_-DN and PKC-_ζ_-WT adenovirus for 48 hours and treated with metformin for 1 hour. The cells were lysed and analyzed by Western blotting. The blot is a representative of 3 blots obtained from 3 individual experiments.
Figure 4
PKC-ζ_–dependent LKB1 phosphorylation of LKB1 at serine 428. A and B, Metformin-activated AMPK is LKB1 dependent in BAEC. Adenoviral overexpression of the kinase-dead LKB1 mutant blocks metformin-induced AMPK activation, whereas LKB1 WT overexpression enhanced the effect of LKB1 in BAEC. The blot is a representative of 5 blots from 5 independent experiments. C and D, Metformin-enhanced LKB1 phosphorylation at serine 428 is PKC-ζ dependent. The blot is a representative of 5 blots from 5 independent experiments (n=5). ♣_P<0.05 (control [Con] vs metformin), †P<0.05 (metformin vs metformin plus PKC-ζ adenovirus). E, LKB1 activity was measured as described in Methods (n=4). F and G, Time course of PKC-ζ, LKB1, and AMPK phosphorylation. BAEC were treated with metformin (1 mmol/L), and the phosphorylation of PKC-ζ, LKB1, and AMPK was detected at the indicated time by Western blot analysis. The blot is a representative of 3 blots from 3 individual experiments. *♣†P<0.05 compared with respective controls.
Figure 5
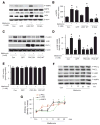
Metformin increases the translocation of LKB1 from nucleus into cytosol in HUVEC. A, Immunocytochemical staining of translocation of LKB1 from nucleus to the cytosols caused by metformin in HUVEC. B and C, Metformin (Met) also increases the amount of LKB1 in the cytosol while it decreases LKB1 in the nuclei in HUVEC. The blot is a representative of 5 blots obtained from 5 independent experiments. Con indicates control. D and E, Metformin increases the serine 428 phosphorylation of LKB1 in both nucleus and cytosol. The blot is a representative of 3 blots obtained from 3 independent experiments.
Figure 6
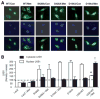
The phosphorylation of LKB1-Ser428 is required for metformin-enhanced translocation of LKB1 and AMPK activation. A, Immunocytochemical staining of metformin (Met)-enhanced LKB1 translocation in A549 cells. The cells were transfected with indicated plasmids, as described in Methods. LKB1 was detected by using a mouse anti-His Tag antibody. The image with Lac-Z was omitted because A549 cells transfected with Lac-Z did not react with the antibody. Con indicates control. B, Western blot detection of LKB1 in A549 cells. The blot is a representative of 5 blots from 5 independent experiments (n=5). ♣P<0.05 (WT vs WT plus metformin), †P<0.05 (WT plus metformin vs WT or S428A plus metformin).
Figure 7
Ser428 phosphorylation of LKB1 is required for metformin-enhanced AMPK activation and coimmunoprecipitation of LKB1 with AMPK. A and B, Metformin activates AMPK in HeLa-S3 overexpressing LKB1-WT but not LKB1-Ser428A mutants. After being transfected with LKB1-WT or kinase-dead LKB1 mutants or the LKB1-Ser428A mutant, HeLa-S3 cells were exposed to metformin (1 mmol/L, 1 hour). AMPK activation was monitored in Western blots with the use of specific antibodies. The blot is a representative of 5 blots from 5 independent experiments. C, Ser428 phosphorylation of LKB1 is required for ONOO−-enhanced AMPK activation. After being transfected with LKB1-WT or LKB1-Ser428A (Ser428 was mutated into alanine, loss of function) or the LKB1-Ser428D (Ser428 replaced by aspartic acid, phosphorylation mimicking), HeLa-S3 cells were exposed to ONOO− (100 _μ_mol/L). AMPK activation was monitored by Western blotting with the use of specific antibodies. The blot is a representative of 5 blots from 5 independent experiments. D, LKB1 activity in LKB1 WT or LKB1-Ser431A mutants. E, Metformin increases the association of LKB1 and AMPK. LKB1 was immunoprecipitated from BAEC, and AMPK was detected in Western blots. Inhibition of PKC-ζ attenuated metformin-enhanced association of LKB1 with AMPK. The blot is a representative of 5 blots from 5 independent experiments. F, Mutation of Ser428 of LKB1 into alanine (S428A) abolishes metformin-enhanced coimmunoprecipitation of LKB1 with AMPK. Metformin increased the coimmunoprecipitation of LKB1 with LKB1 WT but not with LKB1 mutant. The blot is a representative of 3 blots from 3 individual experiments.
Figure 8
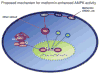
LKB1 nuclear export is required for metformin-enhanced AMPK activation. In the resting state, LKB1 is mainly located in the nucleus, whereas LKB1 activity required that adaptor proteins MO25 and STRAD as well as AMPK-_α_1 (the major isoform of AMPK catalytic units in endothelial cells) be located in the cytosol. Metformin (or ONOO−) activates PI-3 kinase, resulting in PDK-1/2 phosphorylation. PDK-1/2 phosphorylates PKC-ζ, resulting in the translocation of the latter into nucleus as well as cytoplasmic membrane. In the nucleus, activated PKC-ζ phosphorylates LKB1 at serine 428 and likely other sites. Phosphorylated LKB1 is exported from the nucleus via an unknown mechanism into the cytosol. LKB1 is subsequently bound to the preexisting adaptors proteins (STRAD and MO25), which recruit and phosphorylate AMPK at Thr172. AMPK is likely also activated by other stimuli, which either activate PKC-ζ or increase LKB1-Ser428 phosphorylation (PKA or RSK90). Overall, our results suggest that the PI-3 kinase-PDK1/2-PKC-ζ pathway plays a critical role in metformin-enhanced AMPK activation.
Similar articles
- Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells.
Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, Schlattner U, Zou MH. Xie Z, et al. J Biol Chem. 2006 Mar 10;281(10):6366-75. doi: 10.1074/jbc.M511178200. Epub 2006 Jan 9. J Biol Chem. 2006. PMID: 16407220 - Reactive nitrogen species is required for the activation of the AMP-activated protein kinase by statin in vivo.
Choi HC, Song P, Xie Z, Wu Y, Xu J, Zhang M, Dong Y, Wang S, Lau K, Zou MH. Choi HC, et al. J Biol Chem. 2008 Jul 18;283(29):20186-97. doi: 10.1074/jbc.M803020200. Epub 2008 May 12. J Biol Chem. 2008. PMID: 18474592 Free PMC article. Retracted. - Phosphorylation of serine 399 in LKB1 protein short form by protein kinase Cζ is required for its nucleocytoplasmic transport and consequent AMP-activated protein kinase (AMPK) activation.
Zhu H, Moriasi CM, Zhang M, Zhao Y, Zou MH. Zhu H, et al. J Biol Chem. 2013 Jun 7;288(23):16495-16505. doi: 10.1074/jbc.M112.443580. Epub 2013 Apr 23. J Biol Chem. 2013. PMID: 23612973 Free PMC article. - AMP-activated protein kinase in metabolic control and insulin signaling.
Towler MC, Hardie DG. Towler MC, et al. Circ Res. 2007 Feb 16;100(3):328-41. doi: 10.1161/01.RES.0000256090.42690.05. Circ Res. 2007. PMID: 17307971 Review. - Metformin and cancer.
Vallianou NG, Evangelopoulos A, Kazazis C. Vallianou NG, et al. Rev Diabet Stud. 2013 Winter;10(4):228-35. doi: 10.1900/RDS.2013.10.228. Epub 2014 Feb 10. Rev Diabet Stud. 2013. PMID: 24841876 Free PMC article. Review.
Cited by
- Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice.
Li H, Xu M, Lee J, He C, Xie Z. Li H, et al. Am J Physiol Endocrinol Metab. 2012 Nov 15;303(10):E1234-44. doi: 10.1152/ajpendo.00198.2012. Epub 2012 Sep 11. Am J Physiol Endocrinol Metab. 2012. PMID: 22967499 Free PMC article. - AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes.
Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. Eid AA, et al. J Biol Chem. 2010 Nov 26;285(48):37503-12. doi: 10.1074/jbc.M110.136796. Epub 2010 Sep 22. J Biol Chem. 2010. PMID: 20861022 Free PMC article. - Mechanism and role of high density lipoprotein-induced activation of AMP-activated protein kinase in endothelial cells.
Kimura T, Tomura H, Sato K, Ito M, Matsuoka I, Im DS, Kuwabara A, Mogi C, Itoh H, Kurose H, Murakami M, Okajima F. Kimura T, et al. J Biol Chem. 2010 Feb 12;285(7):4387-97. doi: 10.1074/jbc.M109.043869. Epub 2009 Dec 16. J Biol Chem. 2010. PMID: 20018878 Free PMC article. - The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice.
Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L. Saha PK, et al. J Biol Chem. 2010 Dec 24;285(52):40581-92. doi: 10.1074/jbc.M110.176545. Epub 2010 Oct 18. J Biol Chem. 2010. PMID: 20956520 Free PMC article. - Anthocyanin inhibits high glucose-induced hepatic mtGPAT1 activation and prevents fatty acid synthesis through PKCζ.
Guo H, Li D, Ling W, Feng X, Xia M. Guo H, et al. J Lipid Res. 2011 May;52(5):908-22. doi: 10.1194/jlr.M013375. Epub 2011 Feb 22. J Lipid Res. 2011. PMID: 21343633 Free PMC article.
References
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. - PubMed
- Abbasi F, Chu JW, McLaughlin T, Lamendola C, Leary ET, Reaven GM. Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism. 2004;53:159–164. - PubMed
- Verma S, Yao L, Dumont AS, McNeill JH. Metformin treatment corrects vascular insulin resistance in hypertension. J Hypertens. 2000;8:1445–1450. - PubMed
- Katakam PV, Ujhelyi MR, Hoenig M, Miller AW. Metformin improves vascular function in insulin-resistant rats. Hypertension. 2000;35:108–112. - PubMed
- Marfella R, Acampora R, Verrazzo G, Ziccardi P, De RN, Giunta R, Giugliano D. Metformin improves hemodynamic and rheological responses to L-arginine in NIDDM patients. Diabetes Care. 1996;19:934–939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL074399/HL/NHLBI NIH HHS/United States
- R01 HL089920/HL/NHLBI NIH HHS/United States
- R01 HL074399/HL/NHLBI NIH HHS/United States
- HL080499/HL/NHLBI NIH HHS/United States
- HL079584/HL/NHLBI NIH HHS/United States
- R01 HL079584/HL/NHLBI NIH HHS/United States
- 1P20RR024215-01/RR/NCRR NIH HHS/United States
- R01 HL096032/HL/NHLBI NIH HHS/United States
- R01 HL110488/HL/NHLBI NIH HHS/United States
- R01 HL105157/HL/NHLBI NIH HHS/United States
- P20 RR024215/RR/NCRR NIH HHS/United States
- R01 HL080499/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases