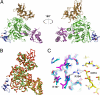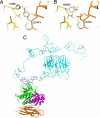Molecular basis for LDL receptor recognition by PCSK9 - PubMed (original) (raw)
Molecular basis for LDL receptor recognition by PCSK9
Hyock Joo Kwon et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9) posttranslationally regulates hepatic low-density lipoprotein receptors (LDLRs) by binding to LDLRs on the cell surface, leading to their degradation. The binding site of PCSK9 has been localized to the epidermal growth factor-like repeat A (EGF-A) domain of the LDLR. Here, we describe the crystal structure of a complex between PCSK9 and the EGF-A domain of the LDLR. The binding site for the LDLR EGF-A domain resides on the surface of PCSK9's subtilisin-like catalytic domain containing Asp-374, a residue for which a gain-of-function mutation (Asp-374-Tyr) increases the affinity of PCSK9 toward LDLR and increases plasma LDL-cholesterol (LDL-C) levels in humans. The binding surface on PCSK9 is distant from its catalytic site, and the EGF-A domain makes no contact with either the C-terminal domain or the prodomain. Point mutations in PCSK9 that altered key residues contributing to EGF-A binding (Arg-194 and Phe-379) greatly diminished binding to the LDLR's extracellular domain. The structure of PCSK9 in complex with the LDLR EGF-A domain defines potential therapeutic target sites for blocking agents that could interfere with this interaction in vivo, thereby increasing LDLR function and reducing plasma LDL-C levels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
The PCSK9:EGF-A complex. (A) PCSK9, with the prodomain (magenta), the subtilisin-like catalytic domain (green), and the C-terminal domain (brown), and the EGF-A domain of LDLR (blue) is represented as a ribbon diagram. The bound calcium ion within the EGF-A domain is shown as a red sphere. (B) Superposition of the PCSK9:EGF-A complex and apo-PCSK9. The PCSK9:EGF-A complex is shown with PCSK9 in green, EGF-A in blue, and bound calcium as a red sphere. Apo-PCSK9 is shown in red. (C) The apo-PCSK9 (Protein Data Bank ID code 2P4E, blue; Protein Data Bank ID code 2PMW, cyan; Protein Data Bank ID code 2QTW, magenta) structures superimposed onto the PCSK9:EGF-A complex [carbon, gray (PCSK9) or yellow (EGF-A); nitrogen, blue; oxygen, red]. Arg-194 from PCSK9 forms a salt bridge with Asp-310 of EGF-A, breaking an intramolecular salt bridge with Glu-197.
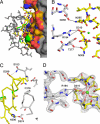
Fig. 2.
Interface between PCSK9 and EGF-A. (A) The surface of PCSK9 buried upon binding of EGF-A. The surface of PCSK9 buried upon binding to EGF-A is colored according to element type: carbon, orange; nitrogen, blue; oxygen, red; sulfur, green. Areas of PCSK9 not involved in binding are colored gray. EGF-A is represented as a stick model. Residues within EGF-A involved in binding are colored according to element type: carbon, yellow; nitrogen, blue; oxygen, red. Residues not involved in binding are colored gray. (B) Interactions between PCSK9 (gray) and EGF-A (yellow) near the calcium (green sphere) binding site of EGF-A. (C) Autocatalysis of PCSK9 is required for binding to EGF-A. Autocatalysis of PCSK9 (gray) between residues 152–153 results in a free N-terminal amine that interacts with EGF-A (yellow). The site of the gain-of-function mutation, Asp-374, on PCSK9 is positioned to interact with His-306 of EGF-A. (D) Calcium coordination within the EGF-A domain after binding to PCSK9. The sigmaA weighted, 2_F_o − _F_c electron density map contoured at 1σ shows the calcium ligands arranged as a pentagonal bipyramid. Asp-310 coordinates calcium (green sphere) and forms a salt bridge with Arg-194 of PCSK9. A water molecule (red sphere) acts as an additional ligand.
Fig. 3.
Mutations in PCSK9 and LDLR. (A) The gain-of-function mutation Asp-374–Tyr in PCSK9 increases binding to LDLR. Asp-374 in PCSK9 modeled as tyrosine (gray) is in position to hydrogen bond to His-306 of LDLR. (B) The FH mutation His-306–Tyr in LDLR. His-306 in LDLR modeled as tyrosine (gray) is in position to hydrogen bond to Asp-374 of PCSK9. (C) Model for full-length LDLR-ECD bound to PCSK9. The EGF-A domain (blue) of the LDLR-ECD (cyan) at acidic pH and the PCKSK9:EGF-A complex were superimposed. PCSK9 (prodomain, magenta; subtilisin-like catalytic domain, green; C-terminal domain, brown) binds on the outside surface of LDLR and would not interfere with the interaction of ligand binding modules R4 and R5 with the β-propeller domain.
Similar articles
- Characterization of the role of EGF-A of low density lipoprotein receptor in PCSK9 binding.
Gu HM, Adijiang A, Mah M, Zhang DW. Gu HM, et al. J Lipid Res. 2013 Dec;54(12):3345-57. doi: 10.1194/jlr.M041129. Epub 2013 Oct 8. J Lipid Res. 2013. PMID: 24103783 Free PMC article. - Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor.
Zhang DW, Garuti R, Tang WJ, Cohen JC, Hobbs HH. Zhang DW, et al. Proc Natl Acad Sci U S A. 2008 Sep 2;105(35):13045-50. doi: 10.1073/pnas.0806312105. Epub 2008 Aug 27. Proc Natl Acad Sci U S A. 2008. PMID: 18753623 Free PMC article. - Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation.
Kosenko T, Golder M, Leblond G, Weng W, Lagace TA. Kosenko T, et al. J Biol Chem. 2013 Mar 22;288(12):8279-8288. doi: 10.1074/jbc.M112.421370. Epub 2013 Feb 11. J Biol Chem. 2013. PMID: 23400816 Free PMC article. - Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.
Urban D, Pöss J, Böhm M, Laufs U. Urban D, et al. J Am Coll Cardiol. 2013 Oct 15;62(16):1401-8. doi: 10.1016/j.jacc.2013.07.056. Epub 2013 Aug 21. J Am Coll Cardiol. 2013. PMID: 23973703 Review. - PCSK9 and LDLR degradation: regulatory mechanisms in circulation and in cells.
Lagace TA. Lagace TA. Curr Opin Lipidol. 2014 Oct;25(5):387-93. doi: 10.1097/MOL.0000000000000114. Curr Opin Lipidol. 2014. PMID: 25110901 Free PMC article. Review.
Cited by
- Proprotein convertase subtilisin/kexin type 9 (PCSK9) and metabolic syndrome: insights on insulin resistance, inflammation, and atherogenic dyslipidemia.
Ferri N, Ruscica M. Ferri N, et al. Endocrine. 2016 Dec;54(3):588-601. doi: 10.1007/s12020-016-0939-0. Epub 2016 Apr 1. Endocrine. 2016. PMID: 27038318 Review. - A new method for measurement of total plasma PCSK9: clinical applications.
Dubuc G, Tremblay M, Paré G, Jacques H, Hamelin J, Benjannet S, Boulet L, Genest J, Bernier L, Seidah NG, Davignon J. Dubuc G, et al. J Lipid Res. 2010 Jan;51(1):140-9. doi: 10.1194/jlr.M900273-JLR200. J Lipid Res. 2010. PMID: 19571328 Free PMC article. - PCSK9 inhibition fails to alter hepatic LDLR, circulating cholesterol, and atherosclerosis in the absence of ApoE.
Ason B, van der Hoorn JW, Chan J, Lee E, Pieterman EJ, Nguyen KK, Di M, Shetterly S, Tang J, Yeh WC, Schwarz M, Jukema JW, Scott R, Wasserman SM, Princen HM, Jackson S. Ason B, et al. J Lipid Res. 2014 Nov;55(11):2370-9. doi: 10.1194/jlr.M053207. Epub 2014 Sep 25. J Lipid Res. 2014. PMID: 25258384 Free PMC article. - β-Estradiol results in a proprotein convertase subtilisin/kexin type 9-dependent increase in low-density lipoprotein receptor levels in human hepatic HuH7 cells.
Starr AE, Lemieux V, Noad J, Moore JI, Dewpura T, Raymond A, Chrétien M, Figeys D, Mayne J. Starr AE, et al. FEBS J. 2015 Jul;282(14):2682-96. doi: 10.1111/febs.13309. Epub 2015 May 18. FEBS J. 2015. PMID: 25913303 Free PMC article. - Plasma Membrane Tetraspanin CD81 Complexes with Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) and Low Density Lipoprotein Receptor (LDLR), and Its Levels Are Reduced by PCSK9.
Le QT, Blanchet M, Seidah NG, Labonté P. Le QT, et al. J Biol Chem. 2015 Sep 18;290(38):23385-400. doi: 10.1074/jbc.M115.642991. Epub 2015 Jul 20. J Biol Chem. 2015. PMID: 26195630 Free PMC article.
References
- Lambert G. Unraveling the functional significance of PCSK9. Curr Opin Lipidol. 2007;18:304–309. - PubMed
- Abifadel M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. - PubMed
- Cohen J, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–165. - PubMed
- Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL-20948/HL/NHLBI NIH HHS/United States
- R01 HL038049/HL/NHLBI NIH HHS/United States
- GM08014/GM/NIGMS NIH HHS/United States
- HL-38049/HL/NHLBI NIH HHS/United States
- P01 HL020948/HL/NHLBI NIH HHS/United States
- T32 GM008014/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous