Isoform-specific functions of phosphoinositide 3-kinases: p110 delta but not p110 gamma promotes optimal allergic responses in vivo - PubMed (original) (raw)
Comparative Study
Isoform-specific functions of phosphoinositide 3-kinases: p110 delta but not p110 gamma promotes optimal allergic responses in vivo
Khaled Ali et al. J Immunol. 2008.
Abstract
The leukocyte-enriched p110gamma and p110delta isoforms of PI3K have been shown to control in vitro degranulation of mast cells induced by cross-linking of the high affinity receptor of IgE (FcepsilonRI). However, the relative contribution of these PI3K isoforms in IgE-dependent allergic responses in vivo is controversial. A side-by-side comparative analysis of the role of p110gamma and p110delta in mast cell function, using genetic approaches and newly developed isoform-selective pharmacologic inhibitors, confirms that both PI3K isoforms play an important role in FcepsilonRI-activated mast cell degranulation in vitro. In vivo, however, only p110delta was found to be required for optimal IgE/Ag-dependent hypersensitivity responses in mice. These observations identify p110delta as a key therapeutic target among PI3K isoforms for allergy- and mast cell-related diseases.
Figures
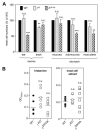
FIGURE 1
Impact of genetic inactivation of p110_γ_ or p110_δ_ on mast cell numbers and vascular permeability responses in vivo. A, Mast numbers in different anatomical locations. Depending on the anatomical location, the units for counting were as follows: ear dermis: per 5 mm length, beginning at the ear tip; stomach: per one complete sagittal section; and back dermis: unit is per 10 high power fields (×40 objective = ×400 magnification). Exclusively, dermal mast cells (those found among dermal collagen) were counted for each mouse; s.c. mast cells (those found among fat cells of the subcutis) were not counted, as thickness of the s.c. fat can vary greatly among animals depending on body condition. Data are presented as mast cell numbers expressed as % of WT (n = 5 for all genotypes). The mast cell distribution in _δ_D910A mice has been published previously (17) and is presented here for comparative purposes. B, Effect of vasoactive stimuli on vascular leakage in PI3K mutant mice. Numbers of mice used were as follows: histamine: WT, _γ_KO, and _δ_D910A, n = 6 each; and mast cell extract: WT, n = 8; _γ_KO, n = 6; and _δ_D910A, n = 8.
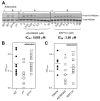
FIGURE 2
Effect of p110_γ_ or p110_δ_ inhibition on adenosine-dependent Akt/PKB phosphorylation in mast cells and on adenosine-dependent vascular permeability. A (Left panel), γ_KO and δ_D910A BMMCs were stimulated with adenosine or vehicle (control) and Akt/PKB phosphorylation assessed by Western blotting, as described in Materials and Methods. A representative blot of two independent experiments is shown. Middle and right panels, BMMCs were pretreated for 30 min with varying concentrations of inhibitors, followed by adenosine stimulation for 1 min and immunoblotted for Akt/PKB (phospho-Ser473 or total). IC50 values were determined by ratiometric analysis of immunoblots, for which Akt/PKB phosphorylation was calculated as the ratio between phosphorylated Akt/PKB and total Akt/PKB for each lane and expressed as the percentage of Akt/PKB phosphorylation in the absence of inhibitor (data not shown). A representative immunoblot of three independent experiments is shown. B, Impact of genetic inactivation of p110_γ or p110_δ on adenosine-induced PCA response in vivo. Number of mice used: WT and γ_KO, n = 10 each; and δ_D910A, n = 11. C, Impact of pharmacological inactivation of p110_γ or p110_δ on adenosine-induced PCA response in vivo. Number of WT mice dosed with AS605240, n = 9 or IC87114, n = 9.
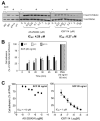
FIGURE 3
Effect of p110_γ_ or p110_δ_ inhibition on SCF-dependent Akt/PKB phosphorylation and adhesion of mast cells. A, (Left panel) _γ_KO and _δ_D910A BMMCs were stimulated with SCF or vehicle (control) and Akt/PKB phosphorylation assessed by western blotting as described in Materials and Methods. A representative blot of two independent experiments is shown. (Middle and right panels), BMMCs were pretreated for 30 min with varying concentrations of inhibitors, followed by stimulation with SCF for 5 min and immunoblotted for Akt/PKB (phospho-Ser473 or total). IC50 values were determined by ratiometric analysis of immunoblots (data not shown), as described in the legend to Fig. 2. A representative immunoblot of three independent experiments is shown. B, Impact of genetic inactivation of PI3K isoforms on SCF-dependent mast cell adhesion. The experiment shown is representative of five independent experiments. C, Impact of pharmacologic inactivation of PI3K isoforms on SCF-dependent mast cell adhesion. Graphs show data from a representative experiment done at least three (AS-2252424) or two (IC87114) times, with identical results.
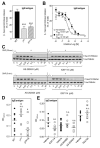
FIGURE 4
Effect of p110_γ_ or p110_δ_ inhibition on IgE-dependent in vitro mast cell degranulation, Akt/PKB phosphorylation, and PCA response in vivo. A, Effect of genetic inactivation of p110_γ_ or p110_δ_ on IgE/Ag-induced BMMC degranulation in vitro. Graph shows the mean ± SD of the following number of independent experiments: WT, n = 10; _δ_D910A, n = 10; and _γ_KO, n = 8; done in quadruplicates. Mean ± SD spontaneous hexosaminidase release for experiments was as follows: WT, 8.23% ± 1.83 (n = 10); _δ_D910A, 11.88% ± 1.9 (n = 10); and γ_KO, 8.0% ± 1.4 (n = 8). B, Effect of isoform-selective PI3K inhibitors on IgE/Ag-induced BMMC degranulation in vitro. Graph shows data from two independent experiments ± SEM. C, Impact of PI3K inhibitors on IgE/Ag-induced Akt/PKB phosphorylation upon 30-s (top panel) and 5-min (bottom panel) stimulation of IgE-sensitized mast cells with Ag. A representative blot from three independent experiments is shown. D, PCA response of WT and gene-targeted mice (WT, γ_KO, and δ_D910A; n = 11 each). E, PCA responses of WT mice treated with PI3K inhibitors. Numbers of mice used were as follows: left panel: IC87114 (p110_δ inhibitor): vehicle, n = 8 and IC87114, n = 9; middle panel: AS-604850 and AS-252424 (p110_γ inhibitors): vehicle, n = 9, AS-604850, n = 10, and AS-252424, n = 8; and right panel: TGX-155 (p110_β inhibitor): vehicle, n = 10 and TGX-155, n = 10.
Similar articles
- Essential role for the p110delta phosphoinositide 3-kinase in the allergic response.
Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Ali K, et al. Nature. 2004 Oct 21;431(7011):1007-11. doi: 10.1038/nature02991. Nature. 2004. PMID: 15496927 - Transient targeting of phosphoinositide 3-kinase acts as a roadblock in mast cells' route to allergy.
Collmann E, Bohnacker T, Marone R, Dawson J, Rehberg M, Stringer R, Krombach F, Burkhart C, Hirsch E, Hollingworth GJ, Thomas M, Wymann MP. Collmann E, et al. J Allergy Clin Immunol. 2013 Oct;132(4):959-68. doi: 10.1016/j.jaci.2013.03.008. Epub 2013 May 14. J Allergy Clin Immunol. 2013. PMID: 23683463 - The phosphoinositide 3-kinase-dependent activation of Btk is required for optimal eicosanoid production and generation of reactive oxygen species in antigen-stimulated mast cells.
Kuehn HS, Swindle EJ, Kim MS, Beaven MA, Metcalfe DD, Gilfillan AM. Kuehn HS, et al. J Immunol. 2008 Dec 1;181(11):7706-12. doi: 10.4049/jimmunol.181.11.7706. J Immunol. 2008. PMID: 19017959 Free PMC article. - The multiple roles of phosphoinositide 3-kinase in mast cell biology.
Kim MS, Rådinger M, Gilfillan AM. Kim MS, et al. Trends Immunol. 2008 Oct;29(10):493-501. doi: 10.1016/j.it.2008.07.004. Epub 2008 Sep 3. Trends Immunol. 2008. PMID: 18775670 Free PMC article. Review. - Phosphoinositide 3-kinase gamma: a key modulator in inflammation and allergy.
Wymann MP, Björklöf K, Calvez R, Finan P, Thomast M, Trifilieff A, Barbier M, Altruda F, Hirsch E, Laffargue M. Wymann MP, et al. Biochem Soc Trans. 2003 Feb;31(Pt 1):275-80. doi: 10.1042/bst0310275. Biochem Soc Trans. 2003. PMID: 12546701 Review.
Cited by
- Inhibition of PI3K promotes dilation of human small airways in a rho kinase-dependent manner.
Koziol-White CJ, Yoo EJ, Cao G, Zhang J, Papanikolaou E, Pushkarsky I, Andrews A, Himes BE, Damoiseaux RD, Liggett SB, Di Carlo D, Kurten RC, Panettieri RA Jr. Koziol-White CJ, et al. Br J Pharmacol. 2016 Sep;173(18):2726-38. doi: 10.1111/bph.13542. Epub 2016 Aug 3. Br J Pharmacol. 2016. PMID: 27352269 Free PMC article. - Inhibition of PI3Kδ improves systemic lupus in mice.
Wang Y, Zhang L, Wei P, Zhang H, Liu C. Wang Y, et al. Inflammation. 2014 Jun;37(3):978-83. doi: 10.1007/s10753-014-9818-0. Inflammation. 2014. PMID: 24445960 - Discovery of dual inhibitors of the immune cell PI3Ks p110delta and p110gamma: a prototype for new anti-inflammatory drugs.
Williams O, Houseman BT, Kunkel EJ, Aizenstein B, Hoffman R, Knight ZA, Shokat KM. Williams O, et al. Chem Biol. 2010 Feb 26;17(2):123-34. doi: 10.1016/j.chembiol.2010.01.010. Chem Biol. 2010. PMID: 20189103 Free PMC article. - The Therapeutic Potential for PI3K Inhibitors in Autoimmune Rheumatic Diseases.
Banham-Hall E, Clatworthy MR, Okkenhaug K. Banham-Hall E, et al. Open Rheumatol J. 2012;6:245-58. doi: 10.2174/1874312901206010245. Epub 2012 Sep 7. Open Rheumatol J. 2012. PMID: 23028409 Free PMC article. - The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (Duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL.
Balakrishnan K, Peluso M, Fu M, Rosin NY, Burger JA, Wierda WG, Keating MJ, Faia K, O'Brien S, Kutok JL, Gandhi V. Balakrishnan K, et al. Leukemia. 2015 Sep;29(9):1811-22. doi: 10.1038/leu.2015.105. Epub 2015 Apr 28. Leukemia. 2015. PMID: 25917267 Free PMC article.
References
- Boyce JA. The biology of the mast cell. Allergy Asthma Proc. 2004;25:27–30. - PubMed
- Wedemeyer J, Galli SJ. Mast cells and basophils in acquired immunity. Br. Med. Bull. 2000;56:936–955. - PubMed
- Nashed BF, Zhang T, Al-Alwan M, Srinivasan G, Halayko AJ, Okkenhaug K, Vanhaesebroeck B, Hayglass KT, Marshall AJ. Role of the phosphoinositide 3-kinase p110δ in generation of type 2 cytokine responses and allergic airway inflammation. Eur. J. Immunol. 2007;37:416–424. - PubMed
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006;6:218–230. - PubMed
- Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu. Rev. Immunol. 2004;22:563–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/C505659/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/C505659/2/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases