The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin - PubMed (original) (raw)
Comparative Study
The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin
Verena Tretter et al. J Neurosci. 2008.
Abstract
Classical benzodiazepine sensitive GABA(A) receptor subtypes, the major mediators of fast synaptic inhibition in the brain are heteropentamers that can be assembled from alpha1-3/5, beta1-3, and gamma2 subunits, but how neurons orchestrate their selective accumulation at synapses remains obscure. We have identified a 10 amino acid hydrophobic motif within the intracellular domain of the alpha2 subunit that regulates the accumulation of GABA(A) receptors at inhibitory synaptic sites on both axon initial segments and dendrites in a mechanism dependent on the inhibitory scaffold protein gephyrin. This motif was sufficient to target CD4 (cluster of differentiation molecule 4) molecules to inhibitory synapses, and was also critical in regulating the direct binding of alpha2 subunits to gephyrin in vitro. Our results thus reveal that the specific accumulation of GABA(A) receptor subtypes containing alpha2 subunits at inhibitory synapses is dependent on their ability to bind gephyrin.
Figures
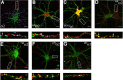
Figure 1.
Analyzing GABAA receptor accumulation on the AIS in hippocampal neurons. A, Endogenous GABAA receptors containing α2 subunits cluster on the AIS. Neurons 18–21 DIV were stained under nonpermeabilized) conditions (without TX-100) with antibodies against an extracellular epitope in the α2 subunit (green) and after permeabilization with 0.05% TX-100 and then with antibodies against VIAAT (blue) and type II Na channels (red). Images were acquired by confocal microscopy. The arrows represent synaptic clusters containing α2 subunits on the AIS. B, C, Differential clustering of recombinant GABAA receptor α2 subunits on the AIS and dendritic processes. 18–21 DIV neurons expressing HAα2 (B) or 9E10α1 (C) subunits were stained with HA or 9E10 antibodies under nonpermeabilized conditions (without TX-100; green), permeabilized with 0.05% TX-100, and then stained with antibodies against type II Na channel (red). The arrows represent clusters of α2 or α1 subunits on the AIS. D, E, Neurons expressing HAα2 were also stained with HA antibody (without TX-100; green) and antibodies against an extracellular epitope on the endogenous α2 subunit (without TX-100; red; D), or permeabilized with 0.05% TX-100 and stained with anti-VIAAT antibodies (red; E). The arrows represent puncta containing HAα2/endogenous α2 subunits and HA/VIAAT in D and E, respectively. F, G, To confirm our results with immunohistochemistry, neurons expressing pHα2 (green) (F) or pHα1 (green) (G) were stained with antibodies against VIAAT (red). In A–G, the bottom panels represent enlargements of the boxed areas in the top panels. Scale bars: A–C, E, 7 μm; D, 10 μm; F, G, 5 μm.
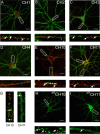
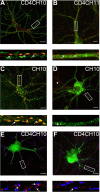
Figure 2.
Analyzing the clustering of GABAA receptor α1/α2 subunit chimeras in hippocampal neurons. A–F, Differential clustering of GABAA receptor chimeras. 18–21 DIV neurons expressing CH1 (A), CH2 (B), CH3 (C), CH4 (D), CH10 (E), or CH11 (F) were stained with HA (CH2, CH11) or 9E10 (CH1, CH3, CH4, and CH10) antibodies under nonpermeabilized conditions (without TX-100; green) and after permeabilization with 0.05% TX-100 with antibodies against the type II Na+ channel (red). Scale bars: 10 μm. Bottom panels represent enlargements of the white boxes in the top panels and the AIS. G, Enlargements of the respective dendrites in yellow boxed areas in E and F are shown. H, I, Live imaging of hippocampal neurons expressing pHluorin-tagged GABAA receptor α1/2 subunit chimeras. 18–21 DIV neurons expressing pHCH10 (H) and pHCH11 (I) were subject to live confocal imaging at 37°C. Bottom panels represent enlargements of the boxed areas in the top panels. Scale bars: 7 μm.
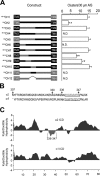
Figure 3.
Analyzing the clustering of α1/2 subunit chimeras on the AIS. A, The structures of CH1-CH12 are indicated in the line diagram. The positions of transmembrane domains are indicated by boxes with sequences derived from the α1 and α2 subunits indicated in black and red, respectively. Chimeras are encoded as follows: CH1 residues 1–306 of the α1 subunit and residues 307–423 of α2; CH2 residues 1–306 of the α2 subunit and residues 306–428 of α1; CH3 residues 307–347 of the α2 subunit substituted for the equivalent amino acids in the α1 subunit; CH4 residues 1–347 of α1 and 348–423 of α2; CH5 residues 1–347 of α2 and 348–428 of α1; CH6 residues 307–329 of the α2 subunit exchanged for the corresponding domain in the α1; CH7 residues 330–347 of the α2 subunit exchanged for the corresponding region in α1; CH8 residues 307–335 of the α2 subunit exchanged for the corresponding domain of α1; CH9 residues 330–335 of the α2 subunit exchanged for the corresponding domain of α1; CH10 residues 336–347 of the α2 subunit exchanged for the corresponding domain of the α1 subunit; CH11 residues 336–348 of the α1 subunit exchanged for the corresponding domain of α2 subunit; and CH12 residues 336–347 deleted from the α2 subunit. Chimeras were modified with either the 9E10 or HA epitopes as indicated. To measure the clustering of these constructs on the AIS, images were recorded from transfected neurons and the AIS was identified by Na+ channel fluorescence and its characteristic morphology. After background subtraction, the number of receptor clusters >0.5 μm2 were counted per 30 μm AIS. Data were then compared with the number of clusters seen with wild-type α2 subunits (control). An asterisk indicates significantly different from control (p < 0.01; n = 11–25 in at least 5 independent transfections). B, An alignment of residues 307–352 of the GABA receptor α1 and α2 subunits. The domain of α2 critical for receptor clustering is shown in red and the corresponding domain of α1 is indicated in blue. The positions of residues 307, 330, 336, and 347 in the α2 subunit are also shown. C, The Kyte and Doolittle hydrophobicity of the major intracellular domains of the α2 (residues 306–390) and α1 (307–391) subunits were determined as indicated. Residues 336–347 of the α2 subunit are highlighted in red.
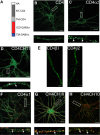
Figure 4.
Clustering of CD4/GABAA receptor chimeras in neurons. A, The structure of GABAA receptor/CD4 chimeras. White box, Extracellular HA epitope; dark gray box, the N terminus of CD4; light gray box, transmembrane domain of the CD4; red box, major intracellular domain of GABAA receptor subunit; blue box, transmembrane 4 of the GABAA receptor subunit. B–F, Images recorded from neurons expressing CD4 (B) CD4α2 (C) and CD4CH11 (D) constructs stained with HA antibody under nonpermeabilized conditions. The arrows represent clusters of CD4 immunoreactivity. E, Processes from neurons expressing CD4β3 and CD4γ2 stained with HA antibody. F, G, Images of neurons expressing CD4α1 (F) and CD4CH10 (G) stained with 9E10 antibody (without membrane permeabilization; green) and after treatment with 0.05% TX-100 with antibodies against VIAAT (red). The arrows represent puncta that contain both VIAAT and CD4 staining. H, Neurons expressing CD4CH10 were stained with HA antibody (without membrane permeabilization; green) and an antibody against endogenous α2 subunits (without membrane permeabilization; red). The arrows represent puncta that contain both HA and endogenous α2 immunoreactivity. In B–H, the bottom panels represent enlargements of the boxed areas in the top panels and the arrows indicate GABAA receptor clusters. Scale bars: B–D, F–H, 10 μm; E, 5 μm.
Figure 5.
Gephyrin mediates the clustering of GABAA receptor chimeras and CD4 expression constructs at synaptic sites. A, B, Selective colocalization of CD4-GABAA receptor clusters with gephyrin. 18–21 DIV hippocampal neurons expressing CD4CH10 (A) or CD4CH11 (B) were stained with HA antibodies (without membrane permeabilization green) against the reporters in these proteins and after membrane permeabilization with antibodies against gephyrin (red) and images then collected by confocal microscopy; arrows indicate puncta that contain both HA and gephyrin immunoreactivity. C, D, Gephyrin expression is critical for the clustering of CH10 in hippocampal neurons. At 18–21 DIV, hippocampal neurons expressing CH10 and a fourfold higher level of a control plasmid (C) or pGEPH1 (D) were stained with 9E10 antibodies (without membrane permeabilization green) and after permeabilization with gephyrin (red) antibodies and subject to confocal microscopy. Colocalizing puncta are indicated by arrows, and the arrowhead represents an untransfected neuron. F, G, The accumulation of CD4CH10 at synaptic sites is dependent on gephyrin expression. At 18–21 DIV, hippocampal neurons expressing control (E) or pGEPH1 (F) were stained with antibodies against the HA epitope (without membrane permeabilization; red) and after membrane permeabilization with antibodies against VIAAT (blue), and endogenous GFP fluorescence is in green. GFP fluorescence has been subtracted from the bottom panels in E and F for clarity. The number of CD4 puncta that were apposed to VIAAT staining on neuronal dendrites were then calculated under control conditions and with pGEPH1 with arrows indicating puncta containing both VIAAT and CD4 immunoreactivity. In A–F, the bottom panels show enlargements of the boxed areas in the top panels arrows. Scale bars: A, C, D, 10 μm; B, E, F, 7 μm.
Figure 6.
Residues of 336–347 mediate the direct binding of gephyrin to the α2 subunit. A, B, Direct binding of gephyrin to the intracellular domain of the α2 subunit. SDS-soluble extracts were prepared from E. coli expressing GST-α2, GST-α2Δ, (residues 330–347) or GST, were overlaid with 35S-methionine-labeled gephyrin in the absence and presence of 0.01% Triton X-100 or immunoblotted with anti-GST antibodies (A). The level of gephyrin binding was corrected for input levels and the level of gephyrin binding to GST-α2Δ was then compared with that seen GST-α2 (control, 100%). Deletion of these residues reduced gephyrin binding to 5.4% of control. The ability of gephyrin to bind to GSTα2 or GST when immobilized on glutathione agarose was measured. Ten micrograms of the respective fusion proteins in the absence of detergent were exposed to unlabeled gephyrin synthesized by in vitro translation and bound material was immunoblotted with anti-gephyrin antibodies. In is 10% of the input used in each assay (B). In, Twenty percent of the starting material. C, Analyzing the binding of gephyrin to the intracellular domains of the receptor β3 and γ2 subunits. SDS-soluble extracts from E. coli expressing GST-α2, β3, and γ2 subunits were overlaid with 35S-methionine gephyrin or immunoblotted with anti-GST antibodies as indicated.
Similar articles
- The residence time of GABA(A)Rs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin.
Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA, Triller A, Schindelin H, Moss SJ. Mukherjee J, et al. J Neurosci. 2011 Oct 12;31(41):14677-87. doi: 10.1523/JNEUROSCI.2001-11.2011. J Neurosci. 2011. PMID: 21994384 Free PMC article. - Molecular basis of the γ-aminobutyric acid A receptor α3 subunit interaction with the clustering protein gephyrin.
Tretter V, Kerschner B, Milenkovic I, Ramsden SL, Ramerstorfer J, Saiepour L, Maric HM, Moss SJ, Schindelin H, Harvey RJ, Sieghart W, Harvey K. Tretter V, et al. J Biol Chem. 2011 Oct 28;286(43):37702-11. doi: 10.1074/jbc.M111.291336. Epub 2011 Aug 31. J Biol Chem. 2011. PMID: 21880742 Free PMC article. - γ-Aminobutyric acid type A (GABAA) receptor α subunits play a direct role in synaptic versus extrasynaptic targeting.
Wu X, Wu Z, Ning G, Guo Y, Ali R, Macdonald RL, De Blas AL, Luscher B, Chen G. Wu X, et al. J Biol Chem. 2012 Aug 10;287(33):27417-30. doi: 10.1074/jbc.M112.360461. Epub 2012 Jun 18. J Biol Chem. 2012. PMID: 22711532 Free PMC article. - Glycinergic transmission.
Kirsch J. Kirsch J. Cell Tissue Res. 2006 Nov;326(2):535-40. doi: 10.1007/s00441-006-0261-x. Epub 2006 Jun 29. Cell Tissue Res. 2006. PMID: 16807723 Review. - The role of GABAAR phosphorylation in the construction of inhibitory synapses and the efficacy of neuronal inhibition.
Vithlani M, Moss SJ. Vithlani M, et al. Biochem Soc Trans. 2009 Dec;37(Pt 6):1355-8. doi: 10.1042/BST0371355. Biochem Soc Trans. 2009. PMID: 19909275 Free PMC article. Review.
Cited by
- The α3 subunit of GABAA receptors promotes formation of inhibitory synapses in the absence of collybistin.
Wagner S, Lee C, Rojas L, Specht CG, Rhee J, Brose N, Papadopoulos T. Wagner S, et al. J Biol Chem. 2021 Jan-Jun;296:100709. doi: 10.1016/j.jbc.2021.100709. Epub 2021 Apr 24. J Biol Chem. 2021. PMID: 33901490 Free PMC article. - A General Procedure to Study Subcellular Models of Transsynaptic Signaling at Inhibitory Synapses.
Lupascu CA, Morabito A, Merenda E, Marinelli S, Marchetti C, Migliore R, Cherubini E, Migliore M. Lupascu CA, et al. Front Neuroinform. 2016 Jun 30;10:23. doi: 10.3389/fninf.2016.00023. eCollection 2016. Front Neuroinform. 2016. PMID: 27445784 Free PMC article. - Complex regulation of Gephyrin splicing is a determinant of inhibitory postsynaptic diversity.
Dos Reis R, Kornobis E, Pereira A, Tores F, Carrasco J, Gautier C, Jahannault-Talignani C, Nitschké P, Muchardt C, Schlosser A, Maric HM, Ango F, Allemand E. Dos Reis R, et al. Nat Commun. 2022 Jun 18;13(1):3507. doi: 10.1038/s41467-022-31264-w. Nat Commun. 2022. PMID: 35717442 Free PMC article. - Diazepam Accelerates GABAAR Synaptic Exchange and Alters Intracellular Trafficking.
Lorenz-Guertin JM, Bambino MJ, Das S, Weintraub ST, Jacob TC. Lorenz-Guertin JM, et al. Front Cell Neurosci. 2019 Apr 26;13:163. doi: 10.3389/fncel.2019.00163. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31080408 Free PMC article. - Identification of a Core Amino Acid Motif within the α Subunit of GABAARs that Promotes Inhibitory Synaptogenesis and Resilience to Seizures.
Nathanson AJ, Zhang Y, Smalley JL, Ollerhead TA, Rodriguez Santos MA, Andrews PM, Wobst HJ, Moore YE, Brandon NJ, Hines RM, Davies PA, Moss SJ. Nathanson AJ, et al. Cell Rep. 2019 Jul 16;28(3):670-681.e8. doi: 10.1016/j.celrep.2019.06.014. Cell Rep. 2019. PMID: 31315046 Free PMC article.
References
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ. GABAA receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neurosci. 2001;4:908–916. - PubMed
- Christie SB, Li RW, Miralles CP, Yang BY, De Blas AL. Clustered and non clustered GABAA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2006;31:1–14. - PubMed
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996a;271:89–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS 051195/NS/NINDS NIH HHS/United States
- R01 NS046478/NS/NINDS NIH HHS/United States
- NS 048045/NS/NINDS NIH HHS/United States
- NS 056359/NS/NINDS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- NS 046478/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials