Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells - PubMed (original) (raw)
Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells
Farida Korobova et al. Mol Biol Cell. 2008 Apr.
Abstract
A role of Arp2/3 complex in lamellipodia is well established, whereas its roles in filopodia formation remain obscure. We addressed this question in neuronal cells, in which motility is heavily based on filopodia, and we found that Arp2/3 complex is involved in generation of both lamellipodia and filopodia in growth cones, and in neuritogenesis, the processes thought to occur largely in Arp2/3 complex-independent manner. Depletion of Arp2/3 complex in primary neurons and neuroblastoma cells by small interfering RNA significantly decreased the F-actin contents and inhibited lamellipodial protrusion and retrograde flow in growth cones, but also initiation and dynamics of filopodia. Using electron microscopy, immunochemistry, and gene expression, we demonstrated the presence of the Arp2/3 complex-dependent dendritic network of actin filaments in growth cones, and we showed that individual actin filaments in filopodia originated at Arp2/3 complex-dependent branch points in lamellipodia, thus providing a mechanistic explanation of Arp2/3 complex functions during filopodia formation. Additionally, Arp2/3 complex depletion led to formation of multiple neurites, erratic pattern of neurite extension, and excessive formation of stress fibers and focal adhesions. Consistent with this phenotype, RhoA activity was increased in Arp2/3 complex-depleted cells, indicating that besides nucleating actin filaments, Arp2/3 complex may influence cell motility by altering Rho GTPase signaling.
Figures
Figure 1.
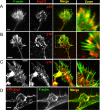
Filopodia initiation in neuronal cells. (A–C) Phase-contrast time-lapse sequences of rat hippocampal neuron (A), differentiated B35 neuroblastoma cell (B), and Xenopus neuron (C). Large panels at left and right show the beginning and the end of the sequence, respectively. Montages in the middle show individual time frames for the boxed regions in the corresponding large panels (B, all; A and C, top) or for other time-lapse sequences (A and C, bottom). Arrows and arrowheads mark individual filopodia formed during the sequence. Filopodia initiation occurs from lamellipodia-like regions and is usually preceded by formation of a small bulge at the leading edge of a growth cone (A–C, top), or at the tip of a secondary neurite (C, bottom), or within a lateral protrusion (A and B, bottom). (D and E) Dynamics of YFP-actin in a growth cone (D) and a lateral protrusion (E) of B35 cells. A corresponding phase contrast sequence is shown for D. Cone-shaped condensation of actin fluorescence precedes filopodia formation (arrows and arrowheads). Time in minutes:seconds. Bars, 5 μm.
Figure 2.
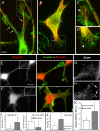
Arp2/3 complex in neuronal cells. (A–C) Localization of Arp2/3 complex (red) by immunostaining with p34-Arc antibody (A) or p16-Arc antibody (B and C) in hippocampal neurons after 4 d in culture (A), or differentiated B35 (B) or NG-108 neuroblastoma cells (C). (D) Expression of YFP-Arp3 in differentiated B35 cells on a background of depletion of endogenous Arp3. Actin (green) is stained by phalloidin (A–D). Boxed regions for A–C are enlarged at right. Arp2/3 complex is present at the periphery of growth cones and variably in the central domains of growth cones and in neuronal shafts. Bars, 2 μm (A, B, and D) or 5 μm (C).
Figure 3.
Structural organization of actin network in neuronal growth cones. EM of differentiated B35 cells (A–C), differentiated NG-108 cells (D), Xenopus neurons (E), or hippocampal neurons (F). (A) Overview of a growth cone. (B and D–F) Periphery of growth cones contains branched actin filaments. Boxed regions are enlarged in insets (B) or below main panels (D–F); branched filaments are highlighted in green. (C) Immunogold (18 nm) staining of growth cones with p34-Arc antibody. Arrows point to gold particles located at branch points. Bars, 1 μm (A), 0.2 μm (B and D–F), or 0.1 μm (C).
Figure 4.
Nascent filopodia contain branched filaments in their roots. Correlative EM of B35 cells. (A) Time-lapse sequence of a growth cone segment shows formation of nascent filopodia (arrows). Black arrow marks formation of a small bulge (0:14), which then forms a filopodium (0:20); the filopodium then becomes partially engulfed by an advancing lamellipodium (0:30–0:36). White arrow marks a small protrusion adjacent to a mature filopodium (0:36). (B) EM of the region shown in A overlaid with a cell contour (yellow line) from 0:14 frame, when the bulge first occurred. Numbered arrows point to corresponding regions in A (0:36); they contain filopodial bundles. Encircled region is enlarged in C. (C–E) High magnifications of nascent filopodia 1 and 2 show branched filaments in their roots (C and D, highlighted in green). Note, that branched filaments in the root of filopodium 2 (C) correspond to the bulge region (B), from which the filopodium emerged. Some actin filaments originated as branches enter the filopodial bundles. Time in minutes:seconds. Bars, 1 μm (A), 0.5 μm (B), or 0.2 μm (D and E).
Figure 5.
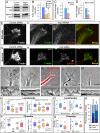
Arp2/3 complex knockdown inhibits lamellipodia and filopodia in B35 neuroblastoma cells and rat hippocampal neurons. (A) Western blotting of undifferentiated B35 cells transfected with control, p34-Arc or Arp3 siRNA. Spectrin is used as loading control. Expression levels of Arp2/3 complex subunits are decreased by ∼50%. (B) Quantification of F-actin in growth cones of differentiated B35 cells transfected with control (n = 25 growth cones), p34-Arc (n = 33) or Arp3 (n = 23) siRNA and stained with fluorescently labeled phalloidin (p < 0.001). (C and D) Quantification of p34-Arc immunofluorescence (C) or F-actin (D) in growth cones of hippocampal neurons transfected with control (n = 29 growth cones) or p34-Arc (n = 15) siRNA (p < 0.001). (E–H) Staining of F-actin by phalloidin and Arp2/3 complex by p16-Arc (F) or p34-Arc (H) antibody in B35 cells (E and F) or hippocampal neurons (G and H) transfected with indicated siRNA. Both actin and Arp2/3 complex components are decreased at the growth cone periphery of knockdown cells (compare phalloidin staining in E and F and in G and H). Images of control and knockdown cells were acquired and processed identically. (I–M) Growth cone dynamics in control (I and L), p34-Arc siRNA-treated (J and M), and rescued (cotransfected with Arp3 siRNA and human YFP-Arp3, K) differentiated B35 cells (I–K) and hippocampal neurons (L and M). Lower panels show kymographs generated along color lines in top panels. Lamellipodia in control (I and L) and rescued (K) growth cones undergo repetitive cycles of protrusion and retraction, whereas siRNA-treated cells (J and M) have relatively quiescent leading edges. Rates of retrograde flow are decreased in knockdown cells (J and M) compared with control (I and L) and rescued cells (K). Examples of slopes used for quantification are shown in kymographs by light blue lines. (N and O) Rates of lamellipodia protrusion (left plots) and retrograde flow (right plots) in differentiated B35 cells (N) and hippocampal neurons (O) transfected as indicated. (P and Q) Filopodia frequency (left plots) and rates of filopodia initiation (right plots) in differentiated B35 cells (P) and hippocampal neurons (Q) transfected as indicated. Plots in N–Q are generated based on analysis of phase contrast movies. Top and bottom of a box indicate 75th and 25th quartiles; whiskers indicate 10th and 90th percentiles; dot is the mean; line is the median. Differences between data sets connected by brackets (N, P) are statistically significant (p < 0.001). Asterisks (O and Q) indicate statistical significance compared with control (p < 0.001). n = 21–49 events for N and O and 11–39 growth cones for P and Q). (R) Filopodia dynamics in control and p34-Arc siRNA-treated differentiated B35 cells. Bar segments represent percentage of time spent by filopodia in protrusion, pause, or retraction phases, as indicated (n = 57–119 filopodia). Bars, 2 μm (E–H) or 5 μm (I–M).
Figure 6.
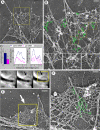
EM of Arp2/3 complex knockdown growth cones of B35 cells. (A) Growth cone of p34-Arc siRNA-treated differentiated cell shows sparse actin network in lamellipodia and few remaining filopodial bundles (compare with Figure 3). (B) Enlarged boxed region from A shows filopodia and lamellipodia in detail. Branched filaments (green) are seen in the sparse lamellipodial network. Many filopodial filaments originate from branch points. Boxed regions in B are further enlarged at bottom without highlighting. (C) Inhibition of lamellipodia in growth cones of differentiated p34-Arc siRNA-treated cells, as revealed by quantification of the lamellipodium width (left), density of actin filament branches (middle), or density of gold particles after immunostaining with p16-Arc antibody (right) (n = 6 cells for each). Branch and immunogold densities are plotted against the distance from the leading edge. (D–F) Correlative EM of nascent filopodia in p34-Arc siRNA-treated differentiated B35 cells. (D) Time-lapse sequence of a growth cone shows formation of a small bulge at the end of the sequence (arrow). (E) EM of the region corresponding to the boxed area in E. Arrow marks a filopodial bundle formed at the position of the bulge. (F) Enlarged boxed region in E. Branched filaments (green) are seen in the root of this thin nascent filopodium. Time in minutes:seconds. Bars, 2 μm (A and D), 0.5 μm (B and E), or 0.2 μm (F).
Figure 7.
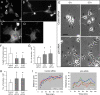
Neuritogenesis is impaired in Arp2/3 complex-depleted B35 cells and hippocampal neurons. (A–D) Phalloidin staining of control (A and B) and p34-Arc-treated (C and D) undifferentiated (A and C) and differentiated (B and D) B35 cells. (E) Mixed population of B35 cells transfected with Cy3-p34-Arc or FITC-control siRNA were imaged immediately (left column) or 12 h (right column) after initiation of differentiation. Numbers mark the same cells at both time points. In the top row, all numbered cells contain control siRNA except cell 2, which contains p34-Arc siRNA, but dies during the movie. In the lower row, all numbered cells contain p34-Arc siRNA. (F and G) Length (F) and number (G) of neurites 48 h after initiation of differentiation in B35 cells treated by control or inhibitory siRNA, as indicated. Asterisks indicate statistical significance compared with control (p < 0.001). n = 152–242 neurites (F) and 63–74 cells (G). (H) Number of neurites in hippocampal neurons 48 h after transfection with control or inhibitory siRNA, as indicated. Asterisks indicate statistical significance compared with control (p < 0.001; n = 56–60). (I) Time course of neurite extension after initiation of differentiation (t = 0) in B35 cells treated with control (left) or p34-Arc (right) siRNA. Each line represents an individual neurite. Dashed lines are curve fitting to a logarithmic function of neurites shown in red in each plot (r, r for plotted variables; p, significance). Bars, 10 μm (A–D) or 5 μm (E).
Figure 8.
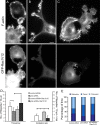
Arp2/3 complex depletion induces stress fibers and activates RhoA. (A–D) Myosin II immunostaining (red) in control (A and C) and Arp3 siRNA-treated (B and D) cells. Actin is labeled by phalloidin (green). Boxed regions in A and B are enlarged in C and D, respectively. Note increased formation of actin bundles in neurites and cell body (arrows) and decreased amount of myosin II puncta in growth cones (arrowheads) of knockdown cells. (E–H) Vinculin immunostaining (red) in control (E and G) and p34-Arc siRNA-treated (F and H) cells. Actin is labeled by phalloidin (green). Boxed regions in E and F are enlarged in G and H, respectively. Note increased formation of focal adhesions in p34-Arc siRNA-treated cells (arrows). (I) Quantification of myosin II immunostaining showing percentage of cells with high contents of myosin II-containing bundles in the cell bodies (left) and percentage of growth cones with peripheral myosin II puncta (right) in B35 cells expressing control and p34-Arc siRNA. (J) Quantification of vinculin immunostaining showing percentage of neurites with focal contacts in B35 cells expressing control and p34-Arc siRNA. (K) RhoA activity in control and p34-Arc siRNA-treated cells. Asterisk indicates statistical significance (p < 0.05; n = 4). Bars, 5 μm (A and B) or 10 μm (E and F).
Figure 9.
Expression of GFP-Rac1V12 in B35 cells. (A–C) Phalloidin staining (top row) of differentiated control (A and B) and p34-Arc siRNA-treated (C) cells expressing GFP-Rac1V12 (bottom row). GFP-Rac1V12 and phalloidin image sets are processed identically, so the brightness of fluorescence reflects the level of Rac1V12 expression or F-actin contents, respectively. Representative cell morphologies are shown. (D) Filopodia frequency (left plot) and rates of filopodia initiation (right plot) in cells transfected with indicated constructs. Values connected by brackets indicate statistical significance (p < 0.01; n = 8–19 cells for each). (E) Filopodia dynamics in cells transfected as indicated. Bar segments represent percentage of time spent by filopodia in protrusion, pause, or retraction phases, as indicated (n = 48–119 filopodia). Bar, 5 μm (A–C).
Figure 10.
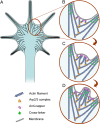
Actin filament organization (A) and the mechanism of filopodia formation (B–D) in neuronal growth cones. (A) Actin filaments (black lines) at the periphery of growth cones are organized into bundles of long filaments in filopodia and branching networks in lamellipodia; these filaments do not form distinct sets, but lamellipodial filaments may branch off of the filopodial filaments and/or merge into filopodial bundles. (B) New filopodia usually form from interfilopodial networks that are rich in Arp2/3 complex (yellow hearts) and branching actin filaments (blue hearts). (C) Arp2/3 complex-nucleated filaments elongate persistently with an assistance of anti-capping molecules (purple rings) and converge to form a bulge-like protrusion at the site of an incipient filopodium. (D) Continued elongation of barbed ends with a concomitant bundling by a cross-linker (green crosses) results in filopodia formation.
Similar articles
- The Role of Rac1 in the Growth Cone Dynamics and Force Generation of DRG Neurons.
Sayyad WA, Fabris P, Torre V. Sayyad WA, et al. PLoS One. 2016 Jan 14;11(1):e0146842. doi: 10.1371/journal.pone.0146842. eCollection 2016. PLoS One. 2016. PMID: 26766136 Free PMC article. - The Arp2/3 complex, UNC-115/abLIM, and UNC-34/Enabled regulate axon guidance and growth cone filopodia formation in Caenorhabditis elegans.
Norris AD, Dyer JO, Lundquist EA. Norris AD, et al. Neural Dev. 2009 Oct 2;4:38. doi: 10.1186/1749-8104-4-38. Neural Dev. 2009. PMID: 19799769 Free PMC article. - Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes.
Steffen A, Faix J, Resch GP, Linkner J, Wehland J, Small JV, Rottner K, Stradal TE. Steffen A, et al. Mol Biol Cell. 2006 Jun;17(6):2581-91. doi: 10.1091/mbc.e05-11-1088. Epub 2006 Apr 5. Mol Biol Cell. 2006. PMID: 16597702 Free PMC article. - Actin dynamics in cell migration.
Schaks M, Giannone G, Rottner K. Schaks M, et al. Essays Biochem. 2019 Oct 31;63(5):483-495. doi: 10.1042/EBC20190015. Essays Biochem. 2019. PMID: 31551324 Free PMC article. Review. - Signaling mechanisms that regulate actin-based motility processes in the nervous system.
Meyer G, Feldman EL. Meyer G, et al. J Neurochem. 2002 Nov;83(3):490-503. doi: 10.1046/j.1471-4159.2002.01185.x. J Neurochem. 2002. PMID: 12390511 Review.
Cited by
- Regulation of the Postsynaptic Compartment of Excitatory Synapses by the Actin Cytoskeleton in Health and Its Disruption in Disease.
Stefen H, Chaichim C, Power J, Fath T. Stefen H, et al. Neural Plast. 2016;2016:2371970. doi: 10.1155/2016/2371970. Epub 2016 Apr 5. Neural Plast. 2016. PMID: 27127658 Free PMC article. Review. - Nerve growth factor promotes reorganization of the axonal microtubule array at sites of axon collateral branching.
Ketschek A, Jones S, Spillane M, Korobova F, Svitkina T, Gallo G. Ketschek A, et al. Dev Neurobiol. 2015 Dec;75(12):1441-61. doi: 10.1002/dneu.22294. Epub 2015 May 27. Dev Neurobiol. 2015. PMID: 25846486 Free PMC article. - High-throughput kinase inhibitor screening reveals roles for Aurora and Nuak kinases in neurite initiation and dendritic branching.
Blazejewski SM, Bennison SA, Liu X, Toyo-Oka K. Blazejewski SM, et al. Sci Rep. 2021 Apr 14;11(1):8156. doi: 10.1038/s41598-021-87521-3. Sci Rep. 2021. PMID: 33854138 Free PMC article. - Two-tiered coupling between flowing actin and immobilized N-cadherin/catenin complexes in neuronal growth cones.
Garcia M, Leduc C, Lagardère M, Argento A, Sibarita JB, Thoumine O. Garcia M, et al. Proc Natl Acad Sci U S A. 2015 Jun 2;112(22):6997-7002. doi: 10.1073/pnas.1423455112. Epub 2015 May 18. Proc Natl Acad Sci U S A. 2015. PMID: 26038554 Free PMC article. - Mechanism of NGF-induced formation of axonal filopodia: NGF turns up the volume, but the song remains the same?
Ketschek A, Spillane M, Gallo G. Ketschek A, et al. Commun Integr Biol. 2011 Jan;4(1):55-8. doi: 10.4161/cib.4.1.13647. Commun Integr Biol. 2011. PMID: 21509179 Free PMC article.
References
- Adams J. C. Roles of fascin in cell adhesion and motility. Curr. Opin. Cell Biol. 2004;16:590–596. - PubMed
- Bear J. E., et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. - PubMed
- Biyasheva A., Svitkina T., Kunda P., Baum B., Borisy G. Cascade pathway of filopodia formation downstream of SCAR. J. Cell Sci. 2004;117:837–848. - PubMed
- Borisy G. G., Svitkina T. M. Actin machinery: pushing the envelope. Curr. Opin. Cell Biol. 2000;12:104–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases