TLR4 links podocytes with the innate immune system to mediate glomerular injury - PubMed (original) (raw)
doi: 10.1681/ASN.2007040395. Epub 2008 Feb 6.
Bernhard Banas, Kelly L Hudkins, Tomasz A Wietecha, Masayuki Iyoda, Elisabeth Bock, Peter Hauser, Jeffrey W Pippin, Stuart J Shankland, Kelly D Smith, Benjamin Stoelcker, Gang Liu, Hermann-Josef Gröne, Bernhard K Krämer, Charles E Alpers
Affiliations
- PMID: 18256364
- PMCID: PMC2390962
- DOI: 10.1681/ASN.2007040395
TLR4 links podocytes with the innate immune system to mediate glomerular injury
Miriam C Banas et al. J Am Soc Nephrol. 2008 Apr.
Abstract
Toll-like receptors (TLR) classically recognize pathogen-associated danger signals but are also activated via endogenous ligands. For evaluation of their role in inflammatory kidney disease, the function of TLR was analyzed in two mouse models of cryoglobulinemic membranoproliferative glomerulonephritis (MPGN; mice transgenic for thymic stromal lymphopoietin [TSLP], with or without deletion of the Fcgamma receptor IIb). Expression of TLR1 through 9 and TLR11 mRNA was detectable in whole kidneys and in isolated glomeruli of wild-type mice, with TLR3 and TLR4 having the highest absolute levels of expression. TLR1, 2, and 4 were increased in TSLP transgenic mice and even higher in TSLP transgenic FcgammaRIIb-deficient mice. TLR5 through 9 and 11 were upregulated to similar degrees in TSLP transgenic and TSLP transgenic FcgammaRIIb-deficient mice. Immunohistochemical studies of nephritic glomeruli localized TLR4 protein to podocytes. Cultured podocytes also expressed TLR4, and stimulation with TLR4-specific ligands resulted in a marked induction of chemokines; this was reduced by specific knockdown of TLR4 with siRNA. Fibrinogen, a potential endogenous TLR4 ligand, was shown to induce a similar profile of chemokines. In conclusion, it was demonstrated that TLR4 is constitutively expressed by podocytes and is upregulated in MPGN, where it may mediate glomerular injury by modulating expression of chemokines; therefore, TLR4 may link podocytes with the innate immune system to mediate MPGN triggered by the deposition of immune complexes.
Figures
Figure 1.
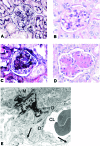
TSLP transgenic mice as a model of MPGN. (A and B) WT mice with normal renal histology at the age of 120 d (A, silver stain; B, periodic acid-Schiff [PAS] stain). (C through E) TSLP transgenic mice at the age of 120 d show typical signs of MPGN as deposition of PAS-positive material (immune deposits including the containing cryoglobulins) in peripheral capillaries and the mesangium (D, PAS stain). The glomerular tuft shows prominent increase of silver staining mesangial matrix (C, silver stain). (E) Electron micrograph of a glomerulus depicting widespread deposition of electron-dense immune complexes in mesangial areas and in subendothelial portions of glomerular capillary walls. Podocytes show focal effacement of foot processes, but many of these are preserved (arrows). There are red blood cells in the capillary lumen. (M, mesangium; D, deposits; CL, capillary lumen). Magnification, ×100.
Figure 2.
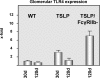
Glomerular TLR4 expression and regulation during MPGN in vivo. Glomeruli were isolated by sieving from (WT), TSLP, and TSLP/FcγRIIb−, respectively, at the given time points. Expression of TLR4 mRNA was analyzed by real-time PCR. At least eight mice were investigated per experimental group and time point. Relative expression values are depicted as means ± SEM.
Figure 3.
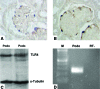
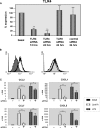
Podocytes express TLR4 in vivo and in vitro. Immunohistochemical localization of TLR4 protein in glomeruli of WT mice (A) and TSLP/FcγRIIb− mice (B). (A and B) Double staining for TLR4 (brown signal) and podocytes (identified by staining for p27kip1; blue signal). TLR4 knockout mice served as control and showed no staining for TLR4 (data not shown). (C and D) Cultured primary murine podocytes show a constitutive expression of TLR4 by Western blot analysis (C) and RT-PCR (D). Size marker (M), lane 1; podocyte total RNA (Podo), lane 2; RT− control, lane 3. Magnification, ×100.
Figure 4.
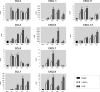
Time course of chemokine induction in podocytes upon activation of TLR4. Cultured podocytes were stimulated with LPS or Lipid A during the shown time range. Relative expression values were analyzed by real-time RT-PCR and normalized to the housekeeping gene cyclophilin. Significant differences are indicated for P < 0.05 (*) or P < 0.01 (**). At least three independent experiments were performed.
Figure 5.
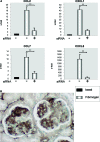
Effects of TLR4-specific siRNA on cultured podocytes. (A) Reduction of TLR4 mRNA in podocytes after transfection with TLR4-specific siRNA for 12 or 24 h (real-time RT-PCR); TLR9-specific siRNA and a nonsilencing siRNA served as controls. (B) Presence of TLR4 protein on the surface of podocytes as detected by FACS analysis. (Left) Naive cells. (Right) Cells incubated with TLR4-specific siRNA for 48 h. (C) Effect of pretreatment with TLR4-specific siRNA on chemokine induction by LPS and Lipid A (real-time RT-PCR). In A and C, significant differences are indicated for P < 0.05 (*) or P < 0.01 (**).
Figure 6.
Fibrinogen activates podocytes via TLR4. (A) Fibrinogen stimulation of cultured podocytes led to an induction of chemokines that was inhibitable by transfection with TLR4-specific siRNA. Significant differences are indicated for P < 0.05 (*) or P < 0.01 (**). (B) Deposition of fibrinogen (brown signal) in glomeruli of MPGN mice shown by immunohistochemistry. Magnification, ×1000.
Comment in
- Are podocytes passive or provocative in proteinuric glomerular pathology?
Tipping PG. Tipping PG. J Am Soc Nephrol. 2008 Apr;19(4):651-3. doi: 10.1681/ASN.2008020156. Epub 2008 Mar 5. J Am Soc Nephrol. 2008. PMID: 18322155 Review. No abstract available.
Similar articles
- Deletion of the fcgamma receptor IIb in thymic stromal lymphopoietin transgenic mice aggravates membranoproliferative glomerulonephritis.
Mühlfeld AS, Segerer S, Hudkins K, Carling MD, Wen M, Farr AG, Ravetch JV, Alpers CE. Mühlfeld AS, et al. Am J Pathol. 2003 Sep;163(3):1127-36. doi: 10.1016/s0002-9440(10)63472-4. Am J Pathol. 2003. PMID: 12937154 Free PMC article. - Overexpression of toll-like receptor 9 correlates with podocyte injury in a murine model of autoimmune membranoproliferative glomerulonephritis.
Masum MA, Ichii O, Hosny Ali Elewa Y, Nakamura T, Otani Y, Hosotani M, Kon Y. Masum MA, et al. Autoimmunity. 2018 Dec;51(8):386-398. doi: 10.1080/08916934.2018.1549234. Epub 2018 Dec 28. Autoimmunity. 2018. PMID: 30592438 - Macrophages are essential contributors to kidney injury in murine cryoglobulinemic membranoproliferative glomerulonephritis.
Guo S, Wietecha TA, Hudkins KL, Kida Y, Spencer MW, Pichaiwong W, Kojima I, Duffield JS, Alpers CE. Guo S, et al. Kidney Int. 2011 Nov;80(9):946-958. doi: 10.1038/ki.2011.249. Epub 2011 Aug 3. Kidney Int. 2011. PMID: 21814168 - Signaling danger: toll-like receptors and their potential roles in kidney disease.
Anders HJ, Banas B, Schlöndorff D. Anders HJ, et al. J Am Soc Nephrol. 2004 Apr;15(4):854-67. doi: 10.1097/01.asn.0000121781.89599.16. J Am Soc Nephrol. 2004. PMID: 15034087 Review. - The role of toll-like receptors in multifactorial mechanisms of early and late renal allotransplant injury, with a focus on the TLR4 receptor and mononuclear cells.
Zmonarski SC, Banasik M, Madziarska K, Mazanowska O, Krajewska M. Zmonarski SC, et al. Adv Clin Exp Med. 2019 Jul;28(7):981-987. doi: 10.17219/acem/94139. Adv Clin Exp Med. 2019. PMID: 30968609 Review.
Cited by
- APOL1 renal risk variants exacerbate podocyte injury by increasing inflammatory stress.
Wakashin H, Heymann J, Roshanravan H, Daneshpajouhnejad P, Rosenberg A, Shin MK, Hoek M, Kopp JB. Wakashin H, et al. BMC Nephrol. 2020 Aug 27;21(1):371. doi: 10.1186/s12882-020-01995-3. BMC Nephrol. 2020. PMID: 32854642 Free PMC article. - Natural compounds improve diabetic nephropathy by regulating the TLR4 signaling pathway.
Wu J, Li K, Zhou M, Gao H, Wang W, Xiao W. Wu J, et al. J Pharm Anal. 2024 Aug;14(8):100946. doi: 10.1016/j.jpha.2024.01.014. Epub 2024 Feb 6. J Pharm Anal. 2024. PMID: 39258172 Free PMC article. Review. - Complement Activation in Nephrotic Glomerular Diseases.
Nell D, Wolf R, Podgorny PM, Kuschnereit T, Kuschnereit R, Dabers T, Stracke S, Schmidt T. Nell D, et al. Biomedicines. 2024 Feb 18;12(2):455. doi: 10.3390/biomedicines12020455. Biomedicines. 2024. PMID: 38398059 Free PMC article. Review. - Role of endotoxemia in causing renal dysfunction in cirrhosis.
Peng JL, Techasatian W, Hato T, Liangpunsakul S. Peng JL, et al. J Investig Med. 2020 Jan;68(1):26-29. doi: 10.1136/jim-2019-001056. Epub 2019 Jul 19. J Investig Med. 2020. PMID: 31324695 Free PMC article. Review. - Class II lupus nephritis with podocyte injury in imiquimod-induced lupus-prone mice.
Murai A, Yamazaki M, Nishihara K, Kito A, Oyama S, Horiba N, Kato A. Murai A, et al. Histol Histopathol. 2022 Jul;37(7):655-664. doi: 10.14670/HH-18-450. Epub 2022 Mar 17. Histol Histopathol. 2022. PMID: 35297032
References
- Dispenzieri A, Gorevic PD: Cryoglobulinemia. Hematol Oncol Clin North Am 13: 1315–1349, 1999 - PubMed
- Fabrizi F, Colucci P, Ponticelli C, Locatelli F: Kidney and liver involvement in cryoglobulinemia. Semin Nephrol 22: 309–318, 2002 - PubMed
- Smith KD, Alpers CE: Pathogenic mechanisms in membranoproliferative glomerulonephritis. Curr Opin Nephrol Hypertens 14: 396–403, 2005 - PubMed
- Aderem A, Ulevitch RJ: Toll-like receptors in the induction of the innate immune response. Nature 406: 782–787, 2000 - PubMed
- Medzhitov R, Janeway C Jr: Innate immunity. N Engl J Med 343: 338–344, 2000 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases