Dendritic cells and cytokines in human inflammatory and autoimmune diseases - PubMed (original) (raw)
Review
Dendritic cells and cytokines in human inflammatory and autoimmune diseases
Patrick Blanco et al. Cytokine Growth Factor Rev. 2008 Feb.
Abstract
Dendritic cells (DCs) produce cytokines and are susceptible to cytokine-mediated activation. Thus, interaction of resting immature DCs with TLR ligands, for example nucleic acids, or with microbes leads to a cascade of pro-inflammatory cytokines and skewing of T cell responses. Conversely, several cytokines are able to trigger DC activation (maturation) via autocrine, for example TNF and plasmacytoid DCs, and paracrine, for example type I IFN and myeloid DCs, pathways. By controlling DC activation, cytokines regulate immune homeostasis and the balance between tolerance and immunity. The increased production and/or bioavailability of cytokines and associated alterations in DC homeostasis have been implicated in various human inflammatory and autoimmune diseases. Targeting these cytokines with biological agents as already is the case with TNF and IL-1 represents a success of immunology and the coming years will expand the range of cytokines as therapeutic targets in autoinflammatory and autoimmune pathology.
Figures
Fig. 1
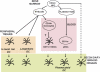
Dendritic cells are composed of subsets. DC progenitors originate from bone marrow CD34+FLT3+ hematopoietic progenitor cells (HPCs). A myeloid pathway generates both Langerhans cells (LCs), found in stratified epithelia such as the skin, and interstitial (int)DCs, found in all other tissues. It also generates mDCs circulating in the blood. Upon inflammation monocytes can yield mDCs. Another pathway generates plasmacytoid DCs (pDCs), which secrete large amounts of IFN-α/β after viral infection. Activated (mature) mDCs and pDCs traffic to secondary lymphoid organs either via afferent lymphatics (mDCs) or blood (pDCs). Langerhans DCs home to cell zones while interstitial DCs home to follicles consistent with their functional specialization, i.e. generation of cellular (Langerhans DCs) and humoral (interstitial DCs) immunity, respectively. The origin of resident lymph node DCs remains to be determined.
Fig. 2
Cytokines and dendritic cell activation. DCs both produce cytokines and are susceptible to cytokine-mediated activation. Thus, exposure to DC activators, for example TLR ligands or microbes, triggers secretion of pro-inflammatory cytokines including type I interferons (IFN), acute phase cytokines such as TNF and IL-6, IL-1 as well as IL-12 family (left panel). Several cytokines are able to trigger DCs activation (maturation) either in autocrine or paracrine fashion including IL-1, TNF, type I IFNs and TSLP (right panel).
Fig. 3
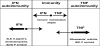
Cross-regulation of TNF and IFN-α in autoimmune diseases. TNF and IFN-α represent opposite vectors (paths) of immune responses. The sum of the vectors yields an equilibrium point, which allows protective immunity when vectors are equal. When one of the vectors prevails beyond a certain threshold, the equilibrium point moves into a zone of autoimmunity: an excess of IFN-α/β is pathogenic in SLE, Sjogren's, dermatomyositis and early stages of psoriasis while excess of TNF is pathogenic in rheumatoid arthritis, inflammatory bowel disease (IBD), Crohn's disease and psoriasis.
Fig. 4
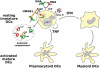
Nucleic acids regulate activation of DC subsets. Interaction of resting immature pDCs with nucleic acids leads to a cascade of cytokine secretion including high amounts of type I IFN as well as TNF. This leads to generation of activated (mature) DCs derived from pDCs, via autocrine TNF, and from immature mDCs, via paracrine type I IFN, both of which drive T and B cell responses. Nucleic acids, abundant in autoimmune diseases such as SLE, can be presented to pDCs via several pathways such as i) immune complexes containing double stranded DNA (green) or single stranded RNA (red) which are taken up via Fc receptors; ii) viruses (for example RNA viruses) taken up via surface receptors; and iii) complexes of nuclear protein HMGB1 bound to DNA-containing immune complexes which are taken up by pDCs via the interaction of HMGB1 with surface protein of the immunoglobulin superfamily RAGE. Ultimately, captured nucleic acids are targeted to endocytic compartments where they bind TLR7 (RNA) or TLR9 (DNA) resulting in activation of signalling pathways that trigger transcription of inflammatory cytokines.
Similar articles
- IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities.
Mohty M, Vialle-Castellano A, Nunes JA, Isnardon D, Olive D, Gaugler B. Mohty M, et al. J Immunol. 2003 Oct 1;171(7):3385-93. doi: 10.4049/jimmunol.171.7.3385. J Immunol. 2003. PMID: 14500632 - Dendritic cell recruitment and activation in autoimmunity.
Sozzani S, Del Prete A, Bosisio D. Sozzani S, et al. J Autoimmun. 2017 Dec;85:126-140. doi: 10.1016/j.jaut.2017.07.012. Epub 2017 Aug 1. J Autoimmun. 2017. PMID: 28774715 Review. - IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells.
Strengell M, Lehtonen A, Matikainen S, Julkunen I. Strengell M, et al. J Leukoc Biol. 2006 Jun;79(6):1279-85. doi: 10.1189/jlb.0905503. Epub 2006 Mar 21. J Leukoc Biol. 2006. PMID: 16551679 - Inflammatory conditions distinctively alter immunological functions of Langerhans-like cells and dendritic cells in vitro.
Said A, Bock S, Müller G, Weindl G. Said A, et al. Immunology. 2015 Feb;144(2):218-30. doi: 10.1111/imm.12363. Immunology. 2015. PMID: 25059418 Free PMC article. - Interferon-α in the generation of monocyte-derived dendritic cells: recent advances and implications for dermatology.
Farkas A, Kemény L. Farkas A, et al. Br J Dermatol. 2011 Aug;165(2):247-54. doi: 10.1111/j.1365-2133.2011.10301.x. Epub 2011 Jun 2. Br J Dermatol. 2011. PMID: 21410666 Review.
Cited by
- Gender roles and traits in stress and health.
Mayor E. Mayor E. Front Psychol. 2015 Jun 9;6:779. doi: 10.3389/fpsyg.2015.00779. eCollection 2015. Front Psychol. 2015. PMID: 26106354 Free PMC article. - Over-expression of a RhoA-specific guanine nucleotide exchange factor, p190RhoGEF, in mouse dendritic cells negatively regulates cellular responses to bacterial lipopolysaccharide.
Seul HJ, Ahn YR, Song HM, Ha YJ, Lee JR. Seul HJ, et al. Mol Cells. 2012 Aug;34(2):159-64. doi: 10.1007/s10059-012-0055-9. Epub 2012 Jul 18. Mol Cells. 2012. PMID: 22814846 Free PMC article. - Multiplexed Detection of Molecular Interactions with DNA Origami Engineered Cells in 3D Collagen Matrices.
Shahhosseini M, Beshay PE, Akbari E, Roki N, Lucas CR, Avendano A, Song JW, Castro CE. Shahhosseini M, et al. ACS Appl Mater Interfaces. 2022 Dec 21;14(50):55307-55319. doi: 10.1021/acsami.2c07971. Epub 2022 Dec 12. ACS Appl Mater Interfaces. 2022. PMID: 36509424 Free PMC article. - TRIF is a critical negative regulator of TLR agonist mediated activation of dendritic cells in vivo.
Seregin SS, Aldhamen YA, Appledorn DM, Aylsworth CF, Godbehere S, Liu CJ, Quiroga D, Amalfitano A. Seregin SS, et al. PLoS One. 2011;6(7):e22064. doi: 10.1371/journal.pone.0022064. Epub 2011 Jul 8. PLoS One. 2011. PMID: 21760953 Free PMC article. - Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β.
Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, Markovics J, Goodsell A, Publicover J, Reichardt L, Jablons D, Wolters P, Hill A, Marks JD, Lou J, Pittet JF, Gauldie J, Baron JL, Nishimura SL. Kitamura H, et al. J Clin Invest. 2011 Jul;121(7):2863-75. doi: 10.1172/JCI45589. Epub 2011 Jun 6. J Clin Invest. 2011. PMID: 21646718 Free PMC article.
References
- Germain RN. An innately interesting decade of research in immunology. Nat Med. 2004;10:1307–20. - PubMed
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. - PubMed
- Sakaguchi S. Naturally arising Foxp3-expressing CD25 + CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. - PubMed
- Shevach EM. Regulatory/suppressor T cells in health and disease. Arthritis Rheum. 2004;50:2721–4. - PubMed
- Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA084512/CA/NCI NIH HHS/United States
- R01 AR050770/AR/NIAMS NIH HHS/United States
- U19 AI057234/AI/NIAID NIH HHS/United States
- R0-1 AR050770-01/AR/NIAMS NIH HHS/United States
- R01 CA078846/CA/NCI NIH HHS/United States
- P50 AR054083/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical