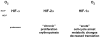Biology of hypoxia-inducible factor-2alpha in development and disease - PubMed (original) (raw)
Review
Biology of hypoxia-inducible factor-2alpha in development and disease
S A Patel et al. Cell Death Differ. 2008 Apr.
Abstract
The transcriptional response to hypoxia is primarily mediated by two hypoxia-inducible factors--HIF-1alpha and HIF-2alpha. While these proteins are highly homologous, increasing evidence suggests they have unique transcriptional targets and differential impact on tumor growth. Furthermore, non-transcriptional effects of the HIF-alpha subunits, including effects on the Notch and c-Myc pathways, contribute to their distinct functions. HIF-2alpha transcriptional targets include genes involved in erythropoiesis, angiogenesis, metastasis, and proliferation. Therefore, HIF-2alpha contributes significantly to both normal physiology as well as tumorigenesis. Here, we summarize the function of HIF-2alpha during development as well as its contribution to pathologic conditions, such as tumors and vascular disease.
Figures
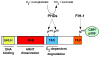
Figure 1
Structure of HIF-2α including the bHLH DNA binding domain, PAS domain and transactivation domains (TADs). Prolyl hydroxylases (PHDs) hydroxylate proline residues 405 and 531 in the oxygen-dependent degradation (ODD) of HIF-2α under normoxic conditions, targeting it for degradation by the proteosome. In addition, hydroxylation of asparagine 847 in the C-terminal TAD by factor-inhibiting HIF (FIH-1) inhibits interaction with the co-activators CBP/p300.
Figure 2
A. Transcription-dependent functions. Ets family transcription factors have been shown to cooperate specifically with HIF-2α to activate select target genes (e.g. PAI-1, EPO, CITED-2). B. Transcription-independent activities. HIF-2α regulates the activity of transcription factors such as Notch and c-Myc, thereby influencing cell proliferation, metabolism and differentiation.
Figure 3
The HIF-α subunits may mediate different adaptive responses to hypoxia. HIF-2α appears to be stabilized at higher O2 levels and displays fewer growth inhibitory properties than HIF-1α Moreover, prolonged accumulation of HIF-2α suggests it could be important for adaptation to chronic hypoxia.
Similar articles
- HIF-2α promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways.
Yan Y, Liu F, Han L, Zhao L, Chen J, Olopade OI, He M, Wei M. Yan Y, et al. J Exp Clin Cancer Res. 2018 Oct 19;37(1):256. doi: 10.1186/s13046-018-0925-x. J Exp Clin Cancer Res. 2018. PMID: 30340507 Free PMC article. - Mild chronic hypoxia-induced HIF-2α interacts with c-MYC through competition with HIF-1α to induce hepatocellular carcinoma cell proliferation.
Mu H, Yu G, Li H, Wang M, Cui Y, Zhang T, Song T, Liu C. Mu H, et al. Cell Oncol (Dordr). 2021 Oct;44(5):1151-1166. doi: 10.1007/s13402-021-00625-w. Epub 2021 Aug 2. Cell Oncol (Dordr). 2021. PMID: 34339013 - Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells.
Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Hu CJ, et al. Mol Cell Biol. 2006 May;26(9):3514-26. doi: 10.1128/MCB.26.9.3514-3526.2006. Mol Cell Biol. 2006. PMID: 16611993 Free PMC article. - Hypoxia inducible factor-2alpha: a critical mediator of aggressive tumor phenotypes.
Qing G, Simon MC. Qing G, et al. Curr Opin Genet Dev. 2009 Feb;19(1):60-6. doi: 10.1016/j.gde.2008.12.001. Epub 2009 Jan 21. Curr Opin Genet Dev. 2009. PMID: 19167211 Free PMC article. Review. - Progress on hypoxia-inducible factor-3: Its structure, gene regulation and biological function (Review).
Yang SL, Wu C, Xiong ZF, Fang X. Yang SL, et al. Mol Med Rep. 2015 Aug;12(2):2411-6. doi: 10.3892/mmr.2015.3689. Epub 2015 Apr 27. Mol Med Rep. 2015. PMID: 25936862 Review.
Cited by
- Activation of HIF2α in kidney proximal tubule cells causes abnormal glycogen deposition but not tumorigenesis.
Fu L, Wang G, Shevchuk MM, Nanus DM, Gudas LJ. Fu L, et al. Cancer Res. 2013 May 1;73(9):2916-25. doi: 10.1158/0008-5472.CAN-12-3983. Epub 2013 Feb 27. Cancer Res. 2013. PMID: 23447580 Free PMC article. - Loss of the SxxSS motif in a human T-cell factor-4 isoform confers hypoxia resistance to liver cancer: an oncogenic switch in Wnt signaling.
Koga H, Tsedensodnom O, Tomimaru Y, Walker EJ, Lee HC, Kim KM, Yano H, Wands JR, Kim M. Koga H, et al. PLoS One. 2012;7(6):e39981. doi: 10.1371/journal.pone.0039981. Epub 2012 Jun 29. PLoS One. 2012. PMID: 22768190 Free PMC article. - Hypoxia-inducible factor-2 is a novel regulator of aberrant CXCL12 expression in multiple myeloma plasma cells.
Martin SK, Diamond P, Williams SA, To LB, Peet DJ, Fujii N, Gronthos S, Harris AL, Zannettino AC. Martin SK, et al. Haematologica. 2010 May;95(5):776-84. doi: 10.3324/haematol.2009.015628. Epub 2009 Dec 16. Haematologica. 2010. PMID: 20015878 Free PMC article. - Mitochondrial reactive oxygen species regulate hypoxic signaling.
Hamanaka RB, Chandel NS. Hamanaka RB, et al. Curr Opin Cell Biol. 2009 Dec;21(6):894-9. doi: 10.1016/j.ceb.2009.08.005. Epub 2009 Sep 24. Curr Opin Cell Biol. 2009. PMID: 19781926 Free PMC article. Review. - RNA-binding proteins implicated in the hypoxic response.
Masuda K, Abdelmohsen K, Gorospe M. Masuda K, et al. J Cell Mol Med. 2009 Sep;13(9A):2759-69. doi: 10.1111/j.1582-4934.2009.00842.x. Epub 2009 Jul 6. J Cell Mol Med. 2009. PMID: 19583805 Free PMC article. Review.
References
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. - PubMed
- Covello KL, Simon MC. HIFs, hypoxia, and vascular development. Curr Top Dev Biol. 2004;62:37–54. - PubMed
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. - PubMed
- Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu Rev Pharmacol Toxicol. 2000;40:519–61. - PubMed
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources