Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice - PubMed (original) (raw)
. 2008 Mar;118(3):1165-75.
doi: 10.1172/JCI33583.
Yanyan Lou, Gregory Lizée, Hong Qin, Shujuan Liu, Brian Rabinovich, Grace J Kim, Yi-Hong Wang, Yang Ye, Andrew G Sikora, Willem W Overwijk, Yong-Jun Liu, Gang Wang, Patrick Hwu
Affiliations
- PMID: 18259609
- PMCID: PMC2230660
- DOI: 10.1172/JCI33583
Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice
Chengwen Liu et al. J Clin Invest. 2008 Mar.
Abstract
A prerequisite for strong adaptive antiviral immunity is the robust initial activation of the innate immune system, which is frequently mediated by TLR-activated plasmacytoid DCs (pDCs). Natural antitumor immunity is often comparatively weak, potentially due to the lack of TLR-mediated activation signals within the tumor microenvironment. To assess whether pDCs are capable of directly facilitating effective antitumor immune responses, mice bearing established subcutaneous B16 melanoma tumors were administered TLR9-activated pDCs directly into the tumor. We found that TLR9-activated pDCs induced robust, spontaneous CTL cross-priming against multiple B16 tumor antigens, leading to the regression of both treated tumors and untreated tumors at distant contralateral sites. This T cell cross-priming was mediated by conventional DCs (cDCs) and was completely dependent upon the early recruitment and activation of NK cells at the tumor site. NK cell recruitment was mediated by CCR5 via chemokines secreted by pDCs, and optimal IFN-gamma production by NK cells was mediated by OX40L expressed by pDCs. Our data thus demonstrated that activated pDCs are capable of initiating effective and systemic antitumor immunity through the orchestration of an immune cascade involving the sequential activation of NK cells, cDCs, and CD8(+) T cells.
Figures
Figure 1. Activated pDC administration induces systemic antitumor activity requiring CD8+ T cells.
Mice bearing 7-day, established subcutaneous B16 tumors were treated by i.t. injection with CpG-activated pDCs, resting pDCs, or saline. Depicted are (A) tumor growth (*P = 0.043 for pDC/CpG versus pDC) and (B) mouse survival as monitored over time following treatment (**P = 0.02 for pDC/CpG versus pDC). (C) Mice bearing subcutaneous B16 tumors in both flanks were treated by i.t. pDC injection in only 1 tumor. Graph depicts growth of untreated, contralateral tumors over time following treatment with CpG-activated or resting pDCs (#P = 0.021 for pDC/CpG versus pDC). (D) Mice bearing single subcutaneous B16 tumors were treated as described in the setting of Ab-mediated CD4+ T cell or CD8+ T cell depletion. Graph depicts tumor growth over time following treatment (##P = 0.009 for pDC/CpG versus pDC/CpG with CD8 depletion). Data shown are expressed as mean ± SEM and are representative of 2 to 3 independent experiments with similar results.
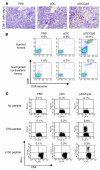
Figure 2. Activated pDCs induce robust cross-priming and tumor infiltration of antigen-specific CTLs.
B16-OVA or B16 tumor–bearing mice were given i.t. pDC or saline injection. (A) Five days later tumor sections were stained for CD8+ T cells. Original magnification, ×100. (B) OVA-specific T cell infiltration of pDC-injected and uninjected tumors, as determined by OVA-tetramer staining. (C) Flow cytometric analysis of intracellular IFN-γ production by splenic CD8+ T cells with no peptide, OVA peptide, or p15E peptide restimulation.
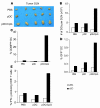
Figure 3. pDC treatment enhances cDC activation, tumor antigen uptake, cross-presentation, and migration to tumor DLN.
B16-OVA or B16-OVA-GFP tumor–bearing mice (5 to 6 mice per group) were injected i.t. with resting pDCs, CpG-activated pDCs, or saline, and 16 to 32 hours later tumor DLN were harvested. (A) Photograph showing tumor DLN of treated mice. (B) Graph depicting absolute numbers of cDCs and pDCs per DLN. (C) Activation status of cDCs and pDCs, as determined by the percentage of CD86hi DCs. (D) Percentages of tumor-associated antigen–positive (GFP+) cDCs and pDCs in tumor DLN. (E) OVA cross-presentation by DLN-derived cDCs and pDCs, as determined by activation of IFN-γ secretion by OT-I T cells. Data are representative of 3 independent experiments with similar results.
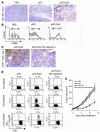
Figure 4. pDC-induced cross-priming of CTLs is dependent on NK infiltration and activation in tumors.
Tumor-bearing mice were given i.t. injection of CpG-activated pDCs, resting pDCs, or saline. (A) NK cell tumor infiltration at treatment day 2, as determined by immunohistochemistry. (B) Expression of CD69 on tumor-infiltrating NK cells. (C) Tumor infiltration by CD8+ T cells at treatment day 5 in mice receiving NK depletion as compared with controls. Original magnification, ×100. (D) Flow cytometric analysis of intracellular IFN-γ production by splenic CD8+ T cells with no peptide, OVA peptide, or p15E peptide restimulation, with or without NK cell depletion. (E) Mice bearing single subcutaneous B16 tumors were treated with pDCs or saline control. If indicated, mice were depleted of NK cells with Ab (*P = 0.027 for pDC/CpG versus pDC/CpG with NK depletion). Data shown are expressed as mean ± SEM and are representative of 3 independent experiments with similar results.
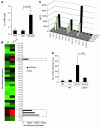
Figure 5. CCR5 is critical for pDC-induced NK cell migration into tumors.
(A) Percentage of tumor-infiltrating NK cells at treatment day 2. (B) Microarray analysis of chemokine transcript expression in resting or CpG-activated pDCs. Signal intensities were normalized to the mean intensity of all the genes represented on the array. (C) Time course analysis of chemokine protein expression in pDCs following activation with CpG. (D) Quantitation of NK cell tumor infiltration 2 days following pDC injection of wild-type or CCR5-deficient mice. Data are expressed as mean ± SD and are representative of 3 experiments with similar results.
Figure 6. pDCs enhance NK cell cytolytic activity through secretion of type I IFNs.
Sorted splenic NK cells were cocultured for 4 h with 51Cr-labeled YAC-1 (A) or B16 (B) tumor cells in the presence of CpG oligonucleotides, resting pDCs, CpG-activated pDCs, or media alone. Specific chromium release was then assessed at different effector/target cell ratios, as shown. (C) Similar experiment as in B, except performed in the presence of blocking Abs, cytokines, or a transwell culture system with a 20:1 effector/target cell ratio, as indicated. (D) Mice were given intravenous pDC injection, and 2 days later NK cells were purified from spleen and assessed for cytotoxicity using B16 tumor cells as targets (*P = 0.001 for pDC/CpG versus pDC). Data are expressed as mean ± SD and are representative of 3 independent experiments with similar results.
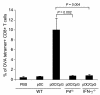
Figure 7. Both perforin and IFN-γ are important for pDC-mediated CTL response.
OVA-tetramer staining of splenocytes derived from pDC-treated B16-OVA tumor–bearing wild-type, perforin-deficient (Prf–/–), or IFN-γ–deficient mice. Data shown are expressed as mean ± SEM and are representative of 3 independent experiments with similar results.
Figure 8. Immune cascade initiated by activated pDCs, culminating in a strong and systemic antitumor immune response.
(i) CpG-activated pDCs produce large amounts of chemokines CCL3, CCL4, and CCL5 within the tumor microenvironment. (ii) NK cells migrate to tumor sites through CCR5-mediated chemotaxis. (iii) Recruited NK cells are activated to produce IFN-γ by pDCs through cytokines and cell-to-cell interactions such as OX40/OX40L. (iv) Activated NK cells initiate tumor cell killing via enhanced cytolytic activity. (v) Tumor-associated antigens released by NK-mediated tumor destruction are taken up by endogenous cDCs, which then (vi) become activated and migrate to tumor DLN. (vii) Cross-presentation of tumor antigens by activated cDCs in DLN leads to effective cross-priming and expansion of tumor antigen–specific T cells. (viii) Infiltration of both treated and untreated tumors by antigen-specific T cells mediates further tumor cell killing and systemic antitumor immunity.
Similar articles
- Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells.
Nierkens S, den Brok MH, Garcia Z, Togher S, Wagenaars J, Wassink M, Boon L, Ruers TJ, Figdor CG, Schoenberger SP, Adema GJ, Janssen EM. Nierkens S, et al. Cancer Res. 2011 Oct 15;71(20):6428-37. doi: 10.1158/0008-5472.CAN-11-2154. Epub 2011 Jul 25. Cancer Res. 2011. PMID: 21788345 Free PMC article. - Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients.
Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, López-Albaitero A, Gibson SP, Gooding WE, Ferrone S, Ferris RL. Srivastava RM, et al. Clin Cancer Res. 2013 Apr 1;19(7):1858-72. doi: 10.1158/1078-0432.CCR-12-2426. Epub 2013 Feb 26. Clin Cancer Res. 2013. PMID: 23444227 Free PMC article. - Activated natural killer cell-mediated immunity is required for the inhibition of tumor metastasis by dendritic cell vaccination.
Kim A, Noh YW, Kim KD, Jang YS, Choe YK, Lim JS. Kim A, et al. Exp Mol Med. 2004 Oct 31;36(5):428-43. doi: 10.1038/emm.2004.55. Exp Mol Med. 2004. PMID: 15557815 - Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity.
Fujii S, Shimizu K, Hemmi H, Steinman RM. Fujii S, et al. Immunol Rev. 2007 Dec;220:183-98. doi: 10.1111/j.1600-065X.2007.00561.x. Immunol Rev. 2007. PMID: 17979847 Review. - Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity.
Lee SC, Srivastava RM, López-Albaitero A, Ferrone S, Ferris RL. Lee SC, et al. Immunol Res. 2011 Aug;50(2-3):248-54. doi: 10.1007/s12026-011-8231-0. Immunol Res. 2011. PMID: 21717064 Free PMC article. Review.
Cited by
- Clinical Aspects and Significance of β-Chemokines, γ-Chemokines, and δ-Chemokines in Molecular Cancer Processes in Acute Myeloid Leukemia (AML) and Myelodysplastic Neoplasms (MDS).
Korbecki J, Bosiacki M, Stasiak P, Snarski E, Brodowska A, Chlubek D, Baranowska-Bosiacka I. Korbecki J, et al. Cancers (Basel). 2024 Sep 24;16(19):3246. doi: 10.3390/cancers16193246. Cancers (Basel). 2024. PMID: 39409868 Free PMC article. Review. - Combination of JAKi and HDACi Exerts Antiangiogenic Potential in Cutaneous T-Cell Lymphoma.
Karagianni F, Piperi C, Valero-Diaz S, Amato C, Vaque JP, Casar B, Papadavid E. Karagianni F, et al. Cancers (Basel). 2024 Sep 17;16(18):3176. doi: 10.3390/cancers16183176. Cancers (Basel). 2024. PMID: 39335148 Free PMC article. - Plasmacytoid dendritic cells at the forefront of anti-cancer immunity: rewiring strategies for tumor microenvironment remodeling.
Monti M, Ferrari G, Gazzurelli L, Bugatti M, Facchetti F, Vermi W. Monti M, et al. J Exp Clin Cancer Res. 2024 Jul 17;43(1):196. doi: 10.1186/s13046-024-03121-9. J Exp Clin Cancer Res. 2024. PMID: 39020402 Free PMC article. Review. - Potent CTLs can be induced against tumor cells in an environment of lower levels of systemic MFG-E8.
Mizote Y, Inoue T, Akazawa T, Kunimasa K, Tamiya M, Kumamoto Y, Tsuda A, Yoshida S, Tatsumi K, Ekawa T, Honma K, Nishino K, Tahara H. Mizote Y, et al. Cancer Sci. 2024 Apr;115(4):1114-1128. doi: 10.1111/cas.16099. Epub 2024 Feb 8. Cancer Sci. 2024. PMID: 38332689 Free PMC article. - Macrophage barrier in the tumor microenvironment and potential clinical applications.
Ji S, Shi Y, Yin B. Ji S, et al. Cell Commun Signal. 2024 Jan 26;22(1):74. doi: 10.1186/s12964-023-01424-6. Cell Commun Signal. 2024. PMID: 38279145 Free PMC article. Review.
References
- Fearon D.T., Locksley R.M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. - PubMed
- Medzhitov R., Janeway C.A., Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. - PubMed
- Megjugorac N.J., et al. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J. Leukoc. Biol. 2004;75:504–514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials