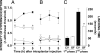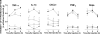Commensal microbiota is fundamental for the development of inflammatory pain - PubMed (original) (raw)
. 2008 Feb 12;105(6):2193-7.
doi: 10.1073/pnas.0711891105. Epub 2008 Feb 11.
D Sachs, V V Costa, C T Fagundes, D Cisalpino, T M Cunha, S H Ferreira, F Q Cunha, T A Silva, J R Nicoli, L Q Vieira, D G Souza, M M Teixeira
Affiliations
- PMID: 18268332
- PMCID: PMC2538897
- DOI: 10.1073/pnas.0711891105
Commensal microbiota is fundamental for the development of inflammatory pain
F A Amaral et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The ability of an individual to sense pain is fundamental for its capacity to adapt to its environment and to avoid damage. The sensation of pain can be enhanced by acute or chronic inflammation. In the present study, we have investigated whether inflammatory pain, as measured by hypernociceptive responses, was modified in the absence of the microbiota. To this end, we evaluated mechanical nociceptive responses induced by a range of inflammatory stimuli in germ-free and conventional mice. Our experiments show that inflammatory hypernociception induced by carrageenan, lipopolysaccharide, TNF-alpha, IL-1beta, and the chemokine CXCL1 was reduced in germ-free mice. In contrast, hypernociception induced by prostaglandins and dopamine was similar in germ-free or conventional mice. Reduction of hypernociception induced by carrageenan was associated with reduced tissue inflammation and could be reversed by reposition of the microbiota or systemic administration of lipopolysaccharide. Significantly, decreased hypernociception in germ-free mice was accompanied by enhanced IL-10 expression upon stimulation and could be reversed by treatment with an anti-IL-10 antibody. Therefore, these results show that contact with commensal microbiota is necessary for mice to develop inflammatory hypernociception. These findings implicate an important role of the interaction between the commensal microbiota and the host in favoring adaptation to environmental stresses, including those that cause pain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Mechanical hypernociception and edema formation induced by injection of carrageenan, LPS, or formalin in germ-free (GF, open symbols) or conventional (CV, filled symbols) mice. (A) Time–response curve of hypernociception induced by i.pl. injection of 30 μl of carrageenan (squares, 100 μg per paw) or 30 μl of saline (circles, vehicle). The hypernociceptive effects were determined at 1, 3, and 5 h after injection. (B) Time-response curve of hypernociception induced by i.pl. injection of 30 μl of LPS (squares, 100 ng per paw) or 30 μl saline (circles, vehicle). (C) Nociceptive responses induced by i.pl. injection of formalin (2%; 30 μl per paw) or 30 μl of saline (vehicle). The nociceptive responses were determined from 0 to 5 min and from 15 to 30 min after formalin injection. Results are expressed by the mean ± SEM of at least six animals per group. *, P < 0.05 when comparing GF and CV mice.
Fig. 2.
Production of TNF-α, IL-1β, and CXCL1 and recruitment of neutrophils after injection of carrageenan in GF (open bars) or CV (filled bars) mice. (A–C) Concentrations of TNF-α (A), IL-1β (B), and CXCL1 (C) in paws injected with 100 μg of Cg or saline (vehicle). (D) Recruitment of neutrophils in soft paw tissue injected with 100 μg of Cg or saline (vehicle). Mice were culled 1, 3, and 5 h after injection, and soft paw tissue was used to measure cytokines by ELISA and neutrophil influx by myeloperoxidase. Results are expressed as the mean ± SEM of five animals per group. *, P < 0.05 when comparing GF and CV mice.
Fig. 3.
Mechanical hypernociception induced by TNF-α, IL-1β, CXCL1, PGE2, and dopamine in GF (open symbols) and CV (filled symbols) mice. Time–response curves of hypernociception induced by i.pl. injection of the mediators (squares) TNF-α (100 pg per paw), IL-1β (100 pg per paw), CXCL1 (10 ng per paw), prostaglandin E2 (PGE2; 100 ng per paw), dopamine (Dopa; 10 μg per paw), or vehicle (circles, 30 μl saline). Results are expressed as the mean ± SEM of six animals per group. Asterisks denote statistically significant differences compared with the control group (P < 0.05).
Fig. 4.
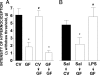
Reversion of antihypernociceptive phenotype of GF (open bars) mice compared with CV (filled bars) ones. (A) Two weeks after feces administration (10 mg/kg; by oral gavage) from conventional or germ-free mice, mechanical hypernociception induced by i.pl. injection carrageenan (100 μg per paw) was evaluated in GF mice and compared with CV and GF vehicle-treated. (B) Then, 48 h after systemic LPS administration (1 mg/kg; s.c.), mechanical hypernociception induced by i.pl. injection of carrageenan (100 μg per paw) was evaluated in GF mice and compared with CV and GF vehicle-treated. The hypernociceptive responses were taken 3 h after carrageenan injection. The results are expressed by the mean ± SEM of at least five animals per group. Asterisks denote statistically significant differences compared with the control group (P < 0.05).
Fig. 5.
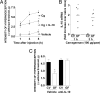
IL-10 mediates the diminished hypernociceptive response of GF mice. (A) CV mice were injected with recombinant murine IL-10 (100 ng per paw, s.c.) before the injection of carrageenan (100 μg per paw). (B) Expression of IL-10 as measured by quantitative PCR in paw skin of CV and GF mice after i.pl. injection of carrageenan (100 μg per paw). (C) GF or CV mice were treated with a monoclonal anti-IL-10 antibody (50 μg per mouse, s.c.) or control antibody (50 μg per mouse) and hypernociception after i.pl. injection of carrageenan (100 μg per paw) evaluated 40 min later. Data are mean ± SEM of measurements of at least five mice per group (except in B) and are from one of two representative experiments performed.
Similar articles
- Crucial role of neutrophils in the development of mechanical inflammatory hypernociception.
Cunha TM, Verri WA Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ. Cunha TM, et al. J Leukoc Biol. 2008 Apr;83(4):824-32. doi: 10.1189/jlb.0907654. Epub 2008 Jan 18. J Leukoc Biol. 2008. PMID: 18203872 - TNF-alpha and IL-1beta mediate inflammatory hypernociception in mice triggered by B1 but not B2 kinin receptor.
Cunha TM, Verri WA Jr, Fukada SY, Guerrero AT, Santodomingo-Garzón T, Poole S, Parada CA, Ferreira SH, Cunha FQ. Cunha TM, et al. Eur J Pharmacol. 2007 Nov 14;573(1-3):221-9. doi: 10.1016/j.ejphar.2007.07.007. Epub 2007 Jul 13. Eur J Pharmacol. 2007. PMID: 17669394 - Role of complement C5a in mechanical inflammatory hypernociception: potential use of C5a receptor antagonists to control inflammatory pain.
Ting E, Guerrero AT, Cunha TM, Verri WA Jr, Taylor SM, Woodruff TM, Cunha FQ, Ferreira SH. Ting E, et al. Br J Pharmacol. 2008 Mar;153(5):1043-53. doi: 10.1038/sj.bjp.0707640. Epub 2007 Dec 17. Br J Pharmacol. 2008. PMID: 18084313 Free PMC article. - Role of cytokines in mediating mechanical hypernociception in a model of delayed-type hypersensitivity in mice.
Cunha TM, Verri WA Jr, Valério DA, Guerrero AT, Nogueira LG, Vieira SM, Souza DG, Teixeira MM, Poole S, Ferreira SH, Cunha FQ. Cunha TM, et al. Eur J Pain. 2008 Nov;12(8):1059-68. doi: 10.1016/j.ejpain.2008.02.003. Epub 2008 Mar 26. Eur J Pain. 2008. PMID: 18372199 - Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development?
Verri WA Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Verri WA Jr, et al. Pharmacol Ther. 2006 Oct;112(1):116-38. doi: 10.1016/j.pharmthera.2006.04.001. Epub 2006 May 30. Pharmacol Ther. 2006. PMID: 16730375 Review.
Cited by
- Experimental colitis-induced visceral hypersensitivity is attenuated by GABA treatment in mice.
Gold MS, Loeza-Alcocer E. Gold MS, et al. Am J Physiol Gastrointest Liver Physiol. 2024 Mar 1;326(3):G252-G263. doi: 10.1152/ajpgi.00012.2023. Epub 2024 Jan 9. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38193198 - Impact of the gut microbiota on rodent models of human disease.
Hansen AK, Hansen CH, Krych L, Nielsen DS. Hansen AK, et al. World J Gastroenterol. 2014 Dec 21;20(47):17727-36. doi: 10.3748/wjg.v20.i47.17727. World J Gastroenterol. 2014. PMID: 25548471 Free PMC article. Review. - The microbiota-gut-brain axis in functional gastrointestinal disorders.
De Palma G, Collins SM, Bercik P. De Palma G, et al. Gut Microbes. 2014 May-Jun;5(3):419-29. doi: 10.4161/gmic.29417. Epub 2014 Jun 12. Gut Microbes. 2014. PMID: 24921926 Free PMC article. Review. - Neonatal Morphine Results in Long-Lasting Alterations to the Gut Microbiome in Adolescence and Adulthood in a Murine Model.
Antoine D, Singh PK, Tao J, Roy S. Antoine D, et al. Pharmaceutics. 2022 Sep 6;14(9):1879. doi: 10.3390/pharmaceutics14091879. Pharmaceutics. 2022. PMID: 36145627 Free PMC article. - The gut microbiota--masters of host development and physiology.
Sommer F, Bäckhed F. Sommer F, et al. Nat Rev Microbiol. 2013 Apr;11(4):227-38. doi: 10.1038/nrmicro2974. Epub 2013 Feb 25. Nat Rev Microbiol. 2013. PMID: 23435359 Review.
References
- Martin HA, Basbaum AI, Kwiat GC, Goetzl EJ, Levine JD. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience. 1987;22:651–659. - PubMed
- Schaible HG, Schmidt RF. Time course of mechanosensitivity changes in articular afferents during a developing experimental arthritis. J Neurophysiol. 1988;60:2180–2195. - PubMed
- Davis KD, Meyer RA, Campbell JN. Chemosensitivity and sensitization of nociceptive afferents that innervate the hairy skin of monkey. J Neurophysiol. 1993;69:1071–1081. - PubMed
- Rueff A, Dray A. Sensitization of peripheral afferent fibres in the in vitro neonatal rat spinal cord-tail by bradykinin and prostaglandins. Neuroscience. 1993;54:527–535. - PubMed
- Vivancos GG, et al. An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res. 2004;37:391–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources