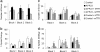Transthyretin protects Alzheimer's mice from the behavioral and biochemical effects of Abeta toxicity - PubMed (original) (raw)
Transthyretin protects Alzheimer's mice from the behavioral and biochemical effects of Abeta toxicity
Joel N Buxbaum et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Cells that have evolved to produce large quantities of secreted proteins to serve the integrated functions of complex multicellular organisms are equipped to compensate for protein misfolding. Hepatocytes and plasma cells have well developed chaperone and proteasome systems to ensure that secreted proteins transit the cell efficiently. The number of neurodegenerative disorders associated with protein misfolding suggests that neurons are particularly sensitive to the pathogenic effects of aggregates of misfolded molecules because those systems are less well developed in this lineage. Aggregates of the amyloidogenic (Abeta(1-42)) peptide play a major role in the pathogenesis of Alzheimer's disease (AD), although the precise mechanism is unclear. In genetic studies examining protein-protein interactions that could constitute native mechanisms of neuroprotection in vivo, overexpression of a WT human transthyretin (TTR) transgene was ameliorative in the APP23 transgenic murine model of human AD. Targeted silencing of the endogenous TTR gene accelerated the development of the neuropathologic phenotype. Intraneuronal TTR was seen in the brains of normal humans and mice and in AD patients and APP23 mice. The APP23 brains showed colocalization of extracellular TTR with Abeta in plaques. Using surface plasmon resonance we obtained in vitro evidence of direct protein-protein interaction between TTR and Abeta aggregates. These findings suggest that TTR is protective because of its capacity to bind toxic or pretoxic Abeta aggregates in both the intracellular and extracellular environment in a chaperone-like manner. The interaction may represent a unique normal host defense mechanism, enhancement of which could be therapeutically useful.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Results of behavioral testing of control and APP23 mice. Separate mixed-sex groups of mice were tested. For younger mice, group sizes were: control WT, two male, five female; m_TTR_−/−, three male, five female; hTTR+, two male, four female; APP23, nine male, nine female; APP23/m_TTR_−/−, nine male, eight female; APP23/hTTR+, five male, four female. For older mice, group sizes were: control WT, seven male, seven female; APP23, six male, six female; APP23 httr, five male, seven female. Mice were tested once daily for 12 days. Sessions were videotaped and scored by an experimenter blind to the mouse genotype. Number of errors per session and strategy used to locate the escape tunnel were recorded. Errors included nose pokes and head deflections over any hole not having the tunnel beneath it. Search strategies were divided into operationally defined categories: (i) random, localized hole searches separated by crossings through the maze center, (ii) serial, systematic hole searches (every or every other hole) in a clockwise or counterclockwise direction, or (iii) spatial, reaching the escape tunnel with both error and distance (number of holes between the first hole visited and the escape tunnel) scores of 3 or less. Data were analyzed in four session blocks using two-way ANOVA with genotype and blocks as variables.
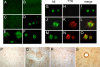
Fig. 2.
Aβ deposition in AD, APP23, and TTR mouse models. Vibratome sections of frontal cortex were treated with formic acid, immunostained with a polyclonal antibody identifying mTTR and hTTR or antibody to Aβ (4G8), and imaged with the confocal microscope (31). (A and B) No amyloid plaques were detected in nontransgenic mice (A) or m_TTR_−/− mice (B). (C) Five-month-old APP23 mice with an intact TTR gene show no plaques. (D) In APP23, m_TTR_−/− mice of the same age plaques are readily detectable. (E and F) Fifteen-month-old APP23 animals (m_TTR_+/+) show large and abundant amyloid plaques (E), which are reduced in APP23 mice expressing hTTR (F). (G–O) Brain sections of APP23 transgenic mice double labeled with antibodies against Aβ (green) and TTR (red) were imaged with the laser scanning confocal microscope. Aβ accumulation in plaques of APP23 (m_TTR_−/−) mice is shown in G–I. There is colocalization of Aβ and mTTR in APP23 (m_TTR_+/+) transgenics (J–L), which is increased in APP23, hTTR transgenics (M–O). (P and Q) There is mild TTR immunolabeling in neuronal cytoplasm of nontransgenic mice (P) and diffuse TTR staining of the plaques and neurons of APP23 transgenic mice (Q). (R) Brains from control nondemented humans show TTR localization in neuronal cell bodies. (S) In the AD brains TTR immunoreactivity was predominantly in vascular amyloid but also seen in neuronal cell bodies. (Magnifications: ×400.)
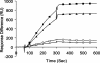
Fig. 3.
Purified recombinant hTTR or mTTR was applied to the Biacore M5 chip surface and exposed to either purified monomer Aβ1–40 or Aβ1–42 or aggregates formed from the same peptides over 7 days before analysis (31). The flow rate was 5 ml/min at 37°C. Ligands were Tetramer TTR Surface (human 4658RU; mouse 5122RU). Analytes were 20 ml of 20 mM Aβ1–42. In reverse experiments the Aβ preparations were applied to the chip and either mTTR or hTTR was allowed to interact in the flow cell. ■, Aβ fibrils on mTTR; ▴, Aβ fibrils on hTTR; □, Aβ monomer on mTTR; ▵, Aβ monomer on hTTR.
Similar articles
- Neuronal production of transthyretin in human and murine Alzheimer's disease: is it protective?
Li X, Masliah E, Reixach N, Buxbaum JN. Li X, et al. J Neurosci. 2011 Aug 31;31(35):12483-90. doi: 10.1523/JNEUROSCI.2417-11.2011. J Neurosci. 2011. PMID: 21880910 Free PMC article. - Transthyretin stability is critical in assisting beta amyloid clearance- Relevance of transthyretin stabilization in Alzheimer's disease.
Alemi M, Silva SC, Santana I, Cardoso I. Alemi M, et al. CNS Neurosci Ther. 2017 Jul;23(7):605-619. doi: 10.1111/cns.12707. Epub 2017 Jun 1. CNS Neurosci Ther. 2017. PMID: 28570028 Free PMC article. - Transthyretin stabilization by iododiflunisal promotes amyloid-β peptide clearance, decreases its deposition, and ameliorates cognitive deficits in an Alzheimer's disease mouse model.
Ribeiro CA, Oliveira SM, Guido LF, Magalhães A, Valencia G, Arsequell G, Saraiva MJ, Cardoso I. Ribeiro CA, et al. J Alzheimers Dis. 2014;39(2):357-70. doi: 10.3233/JAD-131355. J Alzheimers Dis. 2014. PMID: 24169237 - The Role of CSF Transthyretin in Human Alzheimer's Disease: Offense, Defense, or not so Innocent Bystander.
Buxbaum JN. Buxbaum JN. J Integr Neurosci. 2023 Nov 6;22(6):158. doi: 10.31083/j.jin2206158. J Integr Neurosci. 2023. PMID: 38176942 Review. - Transthyretin and the brain re-visited: is neuronal synthesis of transthyretin protective in Alzheimer's disease?
Li X, Buxbaum JN. Li X, et al. Mol Neurodegener. 2011 Nov 23;6:79. doi: 10.1186/1750-1326-6-79. Mol Neurodegener. 2011. PMID: 22112803 Free PMC article. Review.
Cited by
- Roles of constitutively secreted extracellular chaperones in neuronal cell repair and regeneration.
Satapathy S, Wilson MR. Satapathy S, et al. Neural Regen Res. 2023 Apr;18(4):769-772. doi: 10.4103/1673-5374.353483. Neural Regen Res. 2023. PMID: 36204835 Free PMC article. Review. - Thyroid Hormone and Neural Stem Cells: Repair Potential Following Brain and Spinal Cord Injury.
Vancamp P, Butruille L, Demeneix BA, Remaud S. Vancamp P, et al. Front Neurosci. 2020 Aug 26;14:875. doi: 10.3389/fnins.2020.00875. eCollection 2020. Front Neurosci. 2020. PMID: 32982671 Free PMC article. Review. - Vitamin D-binding protein interacts with Aβ and suppresses Aβ-mediated pathology.
Moon M, Song H, Hong HJ, Nam DW, Cha MY, Oh MS, Yu J, Ryu H, Mook-Jung I. Moon M, et al. Cell Death Differ. 2013 Apr;20(4):630-8. doi: 10.1038/cdd.2012.161. Epub 2012 Dec 21. Cell Death Differ. 2013. PMID: 23257976 Free PMC article. - Cerebral amyloid angiopathy-related cardiac injury: Focus on cardiac cell death.
Xu X, Xu H, Zhang Z. Xu X, et al. Front Cell Dev Biol. 2023 Feb 24;11:1156970. doi: 10.3389/fcell.2023.1156970. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36910141 Free PMC article. Review. - Transthyretin protects against A-beta peptide toxicity by proteolytic cleavage of the peptide: a mechanism sensitive to the Kunitz protease inhibitor.
Costa R, Ferreira-da-Silva F, Saraiva MJ, Cardoso I. Costa R, et al. PLoS One. 2008 Aug 6;3(8):e2899. doi: 10.1371/journal.pone.0002899. PLoS One. 2008. PMID: 18682830 Free PMC article.
References
- Schwarzman AL, Goldgaber D. In: The Nature and Origin of Amyloid Fibrils. Bock GR, Goode JA, editors. New York: Wiley; 1996. pp. 146–164.
- Schwarzman AL, et al. Amyloidogenic and anti-amyloidogenic properties of recombinant transthyretin variants. Amyloid. 2004;11:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous