Mammalian septins are required for phagosome formation - PubMed (original) (raw)
Mammalian septins are required for phagosome formation
Yi-Wei Huang et al. Mol Biol Cell. 2008 Apr.
Abstract
Septins are members of a highly conserved family of filamentous proteins that are required in many organisms for the completion of cytokinesis. In addition, septins have been implicated in a number of important cellular processes and have been suggested to have roles in regulating membrane traffic. Given the proposed role of septins in cell membrane dynamics, we investigated the function of septins during FcgammaR-mediated phagocytosis. We show that several septins are expressed in RAW264.7 and J774 mouse macrophage cell lines and that SEPT2 and SEPT11 are colocalized with submembranous actin-rich structures during the early stages of FcgammaR-mediated phagocytosis. In addition, SEPT2 accumulation is seen in primary human neutrophils and in nonprofessional phagocytes. The time course of septin accumulation mirrors actin accumulation and is inhibited by latrunculin and genistein, but not other inhibitors of phagocytosis. Inhibition of septin function by transient expression of the BD3 domain of BORG3, known to cause septin aggregation, or depletion of SEPT2 or SEPT11 by RNAi, significantly inhibited FcgammaR-mediated phagocytosis of IgG-coated latex beads. Interestingly, this occurred without affecting the accumulation of actin or the actin-associated protein coronin-1. These observations show that, although not necessary for actin recruitment, septins are required for efficient FcgammaR-mediated phagocytosis.
Figures
Figure 1.
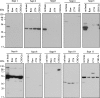
Western blot results of septins in phagocytic cell lines. RAW 264.7, J774, and CHO-IIA cell were harvested, and 10 μg of each cell lysate with 10 μg of rat brain as control were separated by 12% SDS-PAGE before immunoblotting. The blots were probed with rabbit polyclonal antibodies specific to septins 1–6 and 8–11.
Figure 2.
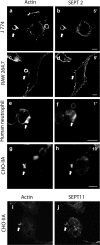
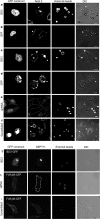
Endogenous SEPT2 and SEPT11 accumulate on phagosomes in different phagocytic cells. Opsonized beads were allowed to attach to the cells for 10 min on ice and then RAW 264.7 (a and b), J 774 (c and d), human neutrophil (e and f), and CHO-IIA cells (g–j) were warmed to 37°C to start phagocytosis for 5, 5, 1, and 10 min, respectively, at 37°C. Cells were then fixed and endogenous SEPT2 was stained with rabbit polyclonal anti-human SEPT2 antibody (b, d, f, and h) or SEPT11 with anti-SEPT11 (j) and detected with Cy3-conjugated donkey anti-rabbit antibody. Actin was stained with Alexa 488-phalloidin (a, c, e, g, and i). The arrows indicate accumulated actin and septin at sites of interaction with opsonized beads in each cell line. Bars, 5 μm.
Figure 3.
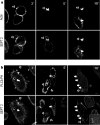
Time course of SEPT2, actin and PLCδ-PH accumulation on phagosomes during phagocytosis in CHO-IIA cells. (a) Opsonized beads were allowed to attach to CHO-IIA cells for 10 min on ice and then warmed up to 37°C to start phagocytosis. At the designated time interval, cells were cooled down on ice to stop phagocytosis. External beads were stained by Cy5-conjugated donkey anti-human IgG antibody for 15 min on ice. Cells were then fixed and stained with rabbit polyclonal anti-human SEPT2 antibody and Cy3-conjugated donkey anti-rabbit secondary antibody for endogenous SEPT2 and Alexa488-phalloidin for actin. (b) CHO-IIA cells were transfected with RFP-PLCδ-PH for 24 h. Phagocytosis was performed and cells were stained as described above. Top panels in a and b are actin and RFP-PLCδ-PH, respectively, and bottom panels are SEPT2. Open arrows point to unsealed phagosomes and solid arrows point to sealed phagosomes. Bars, 5 μm.
Figure 4.
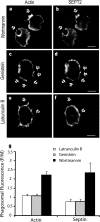
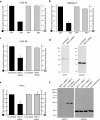
Effects of inhibitors on endogenous SEPT2. CHO-IIA cells were treated with 250 nM wortmannin, or 5 μM latrunculin B for 30 min or 100 μg/ml genistein for 3 h before phagocytosis. Opsonized beads were allowed to attach to the cells for 10 min on ice and then warmed to 37°C to start phagocytosis for 15 min. External beads were stained with Cy5-conjugated donkey anti-human antibody on ice for 15 min. The cells were then fixed and immunostained with Alexa 488- phalloidin for actin and SEPT2 antibody, followed by Cy3-conjugated donkey anti-rabbit antibody for endogenous SEPT2. (a and b) wortmannin, (c and d) genistein, and (e and f) latrunculin treatment. Arrows point to adherent beads. Bars, 5 μm. (g) Quantitation of actin and SEPT2 accumulation after treatment with inhibitors. To quantify the accumulation of actin and SEPT2 at the phagosomal membrane, lines were drawn through the intersections of the phagocytic cup and the contralateral plasma membrane on the colocalized images. The fluorescence intensity of individual pixels was determined using Image J and presented as the ratio of that on the phagosome (P) to that of the normalized plasma membrane (M), hence P/M. In all cases subthreshold intensities were used to ensure that the signals were not saturated. Data are mean ± SE of more than three independent experiments.
Figure 5.
Effects of BD3 and RNAi on endogenous SEPT2 and SEPT11. CHO-IIA cells (a and b) or RAW cells (c and d) were either transfected with GFP-BD3 (a and c) or with GFP as control (b and d) for 36 h. The cells were allowed to ingest IgG opsonized beads for 45 or 30 min at 37°C for CHO-IIA cells or RAW cells, respectively. External beads were stained with Cy5-donkey anti-human IgG for 15 min on ice before immunostaining of the endogenous SEPT2. CHO-IIA cells were transfected with SEPT2 shRNA (e) or with scramble shRNA as control (f) for 84 h. The cells were allowed to ingest IgG opsonized beads for 45 min at 37°C. External beads and endogenous SEPT2 were immunostained as above. Panels from left to right are GFP constructs, endogenous SEPT2, external beads and differential interference contrast (DIC). Open arrows, unsealed phagosomes; solid arrows, sealed phagosomes. Arrowheads point to the transfected cells. (g) As in panel a, CHO-IIA cells were transfected with GFP-BD3 and in this case were stained for SEPT11. (h and i) siRNA specific for SEPT11 (SEPT11 siRNA2) or a scrambled control sequence were transfected into HeLa cells 72 h before the phagocytosis assay, and FcγIIA-GFP was transfected 1 d before the phagocytosis assay. In e and h, transfected cells are also indicated by white dotted lines. Bars, 5 μm.
Figure 6.
BD3 or Sept2 shRNA inhibit Fcγ-IIA receptor mediated phagocytosis. (a and b) Quantification of phagocytic and binding properties in cells transfected with either GFP (control) or with GFP-BD3. Phagocytosis was processed at 37°C for 45 min for CHO-IIA cells (a) and 30 min for RAW cells (b). (c) Quantification of phagocytic and binding properties in CHO-IIA cells transfected with either scramble (control) or SEPT2 shRNA. External and internalized beads were discerned by staining external beads with cy5-donkey anti-human IgG staining for 15 min on ice before permeabilization and immunostaining of the endogenous SEPT2 and internal beads. Phagocytic index and binding index were normalized to control. Data are mean ± SE of at least three independent experiments with at least 50 cells being counted in each case; *p < 0.05 and **p < 0.01. (d) Lysates from CHO-IIA cells transfected with scramble DNA or SEPT2 shRNA were run in 12% SDS-PAGE. The blots were probed with either SEPT2 antibody (left) or GAPDH antibody as loading control (right). (e) Quantification of phagocytosis in HeLa cells depleted of SEPT11. Experiments were performed as in c except that SEPT11 siRNA2 was transfected for 3 d before phagocytic assay and FcγIIA-GFP was transfected in the same culture 1 d before assay. GFP-positive cells were counted for presence of internal and total beads. Similar results were obtained with SEPT11 siRNA1. (f) Lysates from HeLa cells transfected with SEPT11 siRNA1 or 2 or a scrambled control were run in 12% SDS-PAGE. The blots were probed with either SEPT11 antibody (left) or GAPDH antibody as loading control (right).
Figure 7.
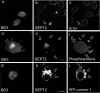
Effects of BD3 on part of the proteins related to Fc receptor signal pathway of CHO-IIA cells. CHO-IIA cells transfected with GFP-BD3 (a, d, and g) or cotransfected with GFP-BD3 and RFP-coronin (i) were allowed to undergo for phagocytosis for 10 min before fixation. Endogenous SEPT2 was immunostained by SEPT2 antibody (b, e, and h), actin by Rhodamine-phalloidin (c) and phosphotyrosine by mouse anti-phosphotyrosine mAb (f). Open arrows, polystyrene beads at unsealed phagocytic cups; Bars, 5 μm.
Similar articles
- Septins regulate bacterial entry into host cells.
Mostowy S, Nam Tham T, Danckaert A, Guadagnini S, Boisson-Dupuis S, Pizarro-Cerdá J, Cossart P. Mostowy S, et al. PLoS One. 2009;4(1):e4196. doi: 10.1371/journal.pone.0004196. Epub 2009 Jan 15. PLoS One. 2009. PMID: 19145258 Free PMC article. - Septins regulate actin organization and cell-cycle arrest through nuclear accumulation of NCK mediated by SOCS7.
Kremer BE, Adang LA, Macara IG. Kremer BE, et al. Cell. 2007 Sep 7;130(5):837-50. doi: 10.1016/j.cell.2007.06.053. Cell. 2007. PMID: 17803907 Free PMC article. - The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology.
Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA. Xie Y, et al. Curr Biol. 2007 Oct 23;17(20):1746-51. doi: 10.1016/j.cub.2007.08.042. Epub 2007 Oct 11. Curr Biol. 2007. PMID: 17935997 - Structural and expression changes of septins in myeloid neoplasia.
Cerveira N, Santos J, Teixeira MR. Cerveira N, et al. Crit Rev Oncog. 2009;15(1-2):91-115. doi: 10.1615/critrevoncog.v15.i1-2.40. Crit Rev Oncog. 2009. PMID: 20136629 Review. - Mammalian septin function in hemostasis and beyond.
Martinez C, Ware J. Martinez C, et al. Exp Biol Med (Maywood). 2004 Dec;229(11):1111-9. doi: 10.1177/153537020422901105. Exp Biol Med (Maywood). 2004. PMID: 15564437 Review.
Cited by
- Septins in Infections: Focus on Viruses.
Henzi T, Lannes N, Filgueira L. Henzi T, et al. Pathogens. 2021 Mar 2;10(3):278. doi: 10.3390/pathogens10030278. Pathogens. 2021. PMID: 33801245 Free PMC article. Review. - Unearthing the role of septins in viral infections.
Khairat JE, Hatta MNA, Abdullah N, Azman AS, Calvin SYM, Syed Hassan S. Khairat JE, et al. Biosci Rep. 2024 Mar 29;44(3):BSR20231827. doi: 10.1042/BSR20231827. Biosci Rep. 2024. PMID: 38372298 Free PMC article. Review. - Arap1 loss causes retinal pigment epithelium phagocytic dysfunction and subsequent photoreceptor death.
Shao A, Lopez AJ, Chen J, Tham A, Javier S, Quiroz A, Frick S, Levine EM, Lloyd KCK, Leonard BC, Murphy CJ, Glaser TM, Moshiri A. Shao A, et al. Dis Model Mech. 2022 Jul 1;15(7):dmm049343. doi: 10.1242/dmm.049343. Epub 2022 Jul 25. Dis Model Mech. 2022. PMID: 35758026 Free PMC article. - The evolution, complex structures and function of septin proteins.
Cao L, Yu W, Wu Y, Yu L. Cao L, et al. Cell Mol Life Sci. 2009 Oct;66(20):3309-23. doi: 10.1007/s00018-009-0087-2. Epub 2009 Jul 14. Cell Mol Life Sci. 2009. PMID: 19597764 Free PMC article. Review. - Septin Organization and Dynamics for Budding Yeast Cytokinesis.
Varela Salgado M, Piatti S. Varela Salgado M, et al. J Fungi (Basel). 2024 Sep 9;10(9):642. doi: 10.3390/jof10090642. J Fungi (Basel). 2024. PMID: 39330402 Free PMC article. Review.
References
- Araki N. Role of microtubules and myosins in Fc gamma receptor-mediated phagocytosis. Front. Biosci. 2006;11:1479–1490. - PubMed
- Beites C. L., Peng X. R., Trimble W. S. Expression and analysis of properties of septin CDCrel-1 in exocytosis. Methods Enzymol. 2001;329:499–510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources