Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2 - PubMed (original) (raw)
Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2
Orbicia Riccio et al. EMBO Rep. 2008 Apr.
Abstract
The crucial role of individual Notch receptors and the mechanism by which they maintain intestinal crypt progenitor cells were assessed by using a series of inducible gut-specific Notch mutant mice. We found that Notch1 and Notch2 receptors function redundantly in the gut, as only simultaneous loss of both receptors results in complete conversion of proliferating crypt progenitors into post-mitotic goblet cells. This conversion correlates with the loss of Hes1 expression and derepression of the cyclin-dependent kinase (CDK) inhibitors p27Kip1 and p57Kip2. We also found that the promoter of both CDK inhibitor genes is occupied by the Notch effector Hes1 in wild-type crypt progenitor cells. Thus, our results indicate that Notch-mediated Hes1 expression contributes to the maintenance of the proliferative crypt compartment of the small intestine by transcriptionally repressing two CDK inhibitors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
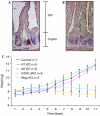
Expression pattern of Notch receptors in the small intestine of wild-type mice. In situ hybridization for (A) N1 and (B) N2 shows expression of both receptors in the crypts of the small intestine. Scale bars, 50 μm. (C) Bodyweight curves of control (N1/N2lox/lox), N1 KO (Notch1lox/lox-vil-Cre-ERT2), N2 KO (Notch2lox/lox-vil-Cre-ERT2), N1N2 dKO (Notch1/Notch2lox/lox-vil-Cre-ERT2) and Rbpj KO (Rbpjlox/lox-vil-Cre-ERT2) post tamoxifen-induced Cre activation. The number of mice analysed is indicated.
Figure 2
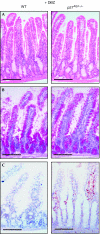
Redundant function of N1 and N2 signalling in the small intestine. Conversion of the proliferative crypt compartment into post-mitotic goblet cells occurs only after simultaneous inactivation of both N1 and N2. Representative sections of the small intestine from control, N1N2 dKO (Notch1/Notch2 lox/lox -vil-Cre-ERT2) and Rbpj KO (Rbpjlox/lox-vil-Cre-ERT2) stained with (A) haematoxylin and eosin, (B) Alcian blue for goblet cell identification, and antibodies against (C) Ki67 and (D) 5-bromodeoxyuridine to identify proliferating crypt progenitors. Scale bars, 50 μm. N1, Notch1; N2, Notch2.
Figure 3
Loss of Notch signalling in the crypt compartment results in loss of Hes1 expression and derepression of the cyclin-dependent kinase inhibitor p27Kip1. (A) Crypt regions of control (blue), N1N2 dKO (Notch1/Notch2lox/lox-vil-Cre-ERT2; green) and Rbpj KO (Rbpjlox/lox-vil- Cre-ERT2; purple) mice were laser microdissected and RNA isolated for quantitative reverse transcription–PCR. Relative levels of messenger RNA of p21 Cip1/Waf1, p27 Kip1 and Hes1 genes normalized with glyceraldehyde phosphate dehydrogenase are shown (logarithmic scale, *P<0.05; results represent the average of four independent experiments). (B–D) Immunohistochemical analysis of sections of the small intestine from (B) control, (C) N1N2 dKO and (D) Rbpj KO stained with an antibody against p27Kip1 bars. (E–G) Immunostaining for Hes1 on sections of the small intestine derived from (E) control, (F) N1N2 dKO and (G) Rbpj KO. The right-hand panels show a higher magnification of crypts from the left-hand panels. Scale bars, 50 μm. (H) Schematic representation of the p27 Kip1 promoter indicating the primers used (a and b) for the chromatin immunoprecipitation (ChIP) analysis. The black rectangle corresponds to a class C Hes1-binding site (CACGCG; Murata et al, 2005). Primers indicated by ‘b', 3 kb upstream of the ATG, were used for control PCR. (I) ChIP assay. Epithelial cells from crypt enrichment preparations were processed for ChIP with an antibody against Hes1 (lane 3) and purified rabbit IgGs as a control (lane 2). PCRs of two distinct regions indicated by ‘a', containing the class C Hes1-binding site, and by ‘b', an Hes1-binding-site-free region, which was used as a negative control, are shown. Lane 1 corresponds to the input DNA.
Figure 4
Loss of p27Kip1 in Notch signalling-inhibited progenitors is not sufficient to maintain their proliferative and undifferentiated phenotype. Treatment of wild type (WT; left) and p27 _Kip1_−/− mice (right) with the γ-secretase inhibitor dibenzazepine (DBZ) followed by morphological and immunohistochemical analysis is shown. Representative small intestine sections stained with (A) haematoxylin and eosin, and (B) periodic acid-Schiff or (C) with an antibody against Ki67 are shown. Scale bars, 100 μm.
Figure 5
Derepression of p57Kip2 expression in the Notch signalling-deficient crypt compartment of the small intestine. (A) Crypt regions of control (blue) and Rbpj KO (purple) mice were laser microdissected followed by RNA isolation and quantitative reverse transcription–PCR for p57Kip2. (B) Schematic representation of the p57 Kip2 promoter indicating the primers used (a and b) for the chromatin immunoprecipitation (ChIP) analysis. The black rectangle corresponds to a class C Hes1-binding site. Primers indicated by ‘b', localizing about 1 kb upstream of the ATG, were used for the control PCR. (C) ChIP assay. Epithelial cells from crypt enrichment preparations were processed for ChIP with an antibody against Hes1 (lane 3) and purified rabbit IgGs as control (lane 2). PCRs of two distinct regions, indicated by ‘a', containing the class C Hes1-binding site, and by ‘b', an unrelated downstream sequence within the p57 promoter used as negative control, are shown. Lane 1 corresponds to input DNA. (D–K) Immunohistochemical analysis of small intestine sections from wild-type (WT) and p27 _Kip1_−/− mice treated with either vehicle alone (−DBZ (dibenzazepine)) or with the γ-secretase inhibitor DBZ (+DBZ) using antibodies against p27Kip1 (D–G) or p57Kip2 (H–K). Note that p57Kip2 is expressed only in the nuclei of Paneth cells (arrowheads) when mice are treated with vehicle alone (H,J). By contrast, γ-secretase-mediated blockage of Notch signalling results in nuclear p57Kip2 staining of crypt progenitor cells (I,K). Scale bars in sections showing p27Kip1 immunostaining correspond to 100 μm, whereas in sections showing p57Kip2 staining, they correspond to 50 μm. (L) Model indicating a possible mechanism by which the Notch signalling pathway promotes the cycling of progenitor cells. Signalling through Notch1 (N1) and Notch2 (N2) in crypt progenitor cells represses the two cyclin-dependent kinase inhibitors p27Kip1 and p57Kip2.
Similar articles
- Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells.
VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud Å, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC. VanDussen KL, et al. Development. 2012 Feb;139(3):488-97. doi: 10.1242/dev.070763. Epub 2011 Dec 21. Development. 2012. PMID: 22190634 Free PMC article. - Genetic evidence that intestinal Notch functions vary regionally and operate through a common mechanism of Math1 repression.
Kim TH, Shivdasani RA. Kim TH, et al. J Biol Chem. 2011 Apr 1;286(13):11427-33. doi: 10.1074/jbc.M110.188797. Epub 2011 Jan 31. J Biol Chem. 2011. PMID: 21282114 Free PMC article. - Antagonistic regulation of p57kip2 by Hes/Hey downstream of Notch signaling and muscle regulatory factors regulates skeletal muscle growth arrest.
Zalc A, Hayashi S, Auradé F, Bröhl D, Chang T, Mademtzoglou D, Mourikis P, Yao Z, Cao Y, Birchmeier C, Relaix F. Zalc A, et al. Development. 2014 Jul;141(14):2780-90. doi: 10.1242/dev.110155. Development. 2014. PMID: 25005473 - Notch-Hes1 pathway contributes to the cochlear prosensory formation potentially through the transcriptional down-regulation of p27Kip1.
Murata J, Ohtsuka T, Tokunaga A, Nishiike S, Inohara H, Okano H, Kageyama R. Murata J, et al. J Neurosci Res. 2009 Dec;87(16):3521-34. doi: 10.1002/jnr.22169. J Neurosci Res. 2009. PMID: 19598246 - Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration.
Carulli AJ, Keeley TM, Demitrack ES, Chung J, Maillard I, Samuelson LC. Carulli AJ, et al. Dev Biol. 2015 Jun 1;402(1):98-108. doi: 10.1016/j.ydbio.2015.03.012. Epub 2015 Mar 30. Dev Biol. 2015. PMID: 25835502 Free PMC article.
Cited by
- The Intestinal Epithelium - Fluid Fate and Rigid Structure From Crypt Bottom to Villus Tip.
Bonis V, Rossell C, Gehart H. Bonis V, et al. Front Cell Dev Biol. 2021 May 20;9:661931. doi: 10.3389/fcell.2021.661931. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095127 Free PMC article. Review. - The regulatory niche of intestinal stem cells.
Sailaja BS, He XC, Li L. Sailaja BS, et al. J Physiol. 2016 Sep 1;594(17):4827-36. doi: 10.1113/JP271931. Epub 2016 Jul 28. J Physiol. 2016. PMID: 27060879 Free PMC article. Review. - Plasticity of Paneth cells and their ability to regulate intestinal stem cells.
Mei X, Gu M, Li M. Mei X, et al. Stem Cell Res Ther. 2020 Aug 12;11(1):349. doi: 10.1186/s13287-020-01857-7. Stem Cell Res Ther. 2020. PMID: 32787930 Free PMC article. Review. - Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells.
VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud Å, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC. VanDussen KL, et al. Development. 2012 Feb;139(3):488-97. doi: 10.1242/dev.070763. Epub 2011 Dec 21. Development. 2012. PMID: 22190634 Free PMC article. - Major signaling pathways in intestinal stem cells.
Vanuytsel T, Senger S, Fasano A, Shea-Donohue T. Vanuytsel T, et al. Biochim Biophys Acta. 2013 Feb;1830(2):2410-26. doi: 10.1016/j.bbagen.2012.08.006. Epub 2012 Aug 16. Biochim Biophys Acta. 2013. PMID: 22922290 Free PMC article. Review.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284: 770–776 - PubMed
- Aster JC (2005) Deregulated NOTCH signaling in acute T-cell lymphoblastic leukemia/lymphoma: new insights, questions, and opportunities. Int J Hematol 82: 295–301 - PubMed
- Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, Lewis J (2005) Delta–Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 132: 1093–1104 - PubMed
- el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S (2004) Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39: 186–193 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous