Midkine is a NF-kappaB-inducible gene that supports prostate cancer cell survival - PubMed (original) (raw)
Midkine is a NF-kappaB-inducible gene that supports prostate cancer cell survival
Zongbing You et al. BMC Med Genomics. 2008.
Abstract
Background: Midkine is a heparin-binding growth factor that is over-expressed in various human cancers and plays important roles in cell transformation, growth, survival, migration, and angiogenesis. However, little is known about the upstream factors and signaling mechanisms that regulate midkine gene expression.
Methods: Two prostate cancer cell lines LNCaP and PC3 were studied for their expression of midkine. Induction of midkine expression in LNCaP cells by serum, growth factors and cytokines was determined by Western blot analysis and/or real-time quantitative reverse-transcription - polymerase chain reaction (RT-PCR). The cell viability was determined by the trypan blue exclusion assay when the LNCaP cells were treated with tumor necrosis factor alpha (TNFalpha) and/or recombinant midkine. When the LNCaP cells were treated with recombinant midkine, activation of intracellular signalling pathways was determined by Western blot analysis. Prostate tissue microarray slides containing 129 cases (18 normal prostate tissues, 40 early stage cancers, and 71 late stage cancers) were assessed for midkine expression by immunohistochemical staining.
Results: We identified that fetal bovine serum, some growth factors (epidermal growth factor, androgen, insulin-like growth factor-I, and hepatocyte growth factor) and cytokines (TNFalpha and interleukin-1beta) induced midkine expression in a human prostate cancer cell line LNCaP cells. TNFalpha also induced midkine expression in PC3 cells. TNFalpha was the strongest inducer of midkine expression via nuclear factor-kappa B pathway. Midkine partially inhibited TNFalpha-induced apoptosis in LNCaP cells. Knockdown of endogenous midkine expression by small interfering RNA enhanced TNFalpha-induced apoptosis in LNCaP cells. Midkine activated extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase pathways in LNCaP cells. Furthermore, midkine expression was significantly increased in late stage prostate cancer, which coincides with previously reported high serum levels of TNFalpha in advanced prostate cancer.
Conclusion: These findings provide the first demonstration that midkine expression is induced by certain growth factors and cytokines, particularly TNFalpha, which offers new insight into understanding how midkine expression is increased in the late stage prostate cancer.
Figures
Figure 1
Midkine partially inhibited TNFα-induced apoptosis in LNCaP cells. A. LNCaP cells in triplicate groups were not treated (as control) or treated for 4 days with 20 ng/ml TNFα, without or with 0.1 or 1 μg/ml exogenous midkine; the living cell number was counted by the trypan blue exclusion assay; the cell survival was calculated as (the living cell number of treated group ÷ the living cell number of untreated control group); the data were presented as mean ± standard deviation; *P < 0.05 compared to TNFα alone. B. LNCaP cells were transfected with the mixtures of midkine specific siRNA/Lipofectamine™ 2000, control-siRNA/Lipofectamine™ 2000, or Lipofectamine™ 2000 only (mock transfection), or no transfection as an additional control; the final concentrations used were 100 nM of siRNA or control-siRNA, and 5 μl/ml of Lipofectamine™ 2000; four h after transfection, the cells were changed into serum-free DMEM without or with 20 ng/ml TNFα; two days later, the medium supernatants were analyzed for midkine expression by Western blot. C. LNCaP cells in triplicate groups were treated as described in B; the cell survival after 2-days' treatment with 20 ng/ml TNFα was determined by the trypan blue exclusion assay as described in A; the data were presented as mean ± standard deviation; *P < 0.05 compared to the other three groups. D. LNCaP cells were treated as described in B; 16 h after treatment with 20 ng/ml TNFα, 20 μM MR-(DEVD)2 were added to the cells and incubated for 1 h, followed by addition of 1 μg/ml Hoechst 33342 for another 15 min; the red fluorescence [emitted by the cleaved MR-(DEVD)2 indicating activation of caspase-3] and blue fluorescent nuclei (stained by Hoechst 33342 to illustrate total cell number) were captured by a fluorescent microscope; original magnification: × 100.
Figure 2
Midkine expression was induced by FBS, growth factors and cytokines. A. LNCaP cells were cultured in serum-free DMEM and treated for 48 h with 10% FBS and the indicated agents: 10 ng/ml insulin, 10 ng/ml IGF-I, 10 ng/ml EGF, 10 ng/ml HGF, 10 ng/ml bFGF, 20 ng/ml T3, 10 nM R1881, 10 nM DHT, 10 ng/ml TNFα, 10 ng/ml IL-1β, 50 ng/ml IL-6, 50 ng/ml IL-17, and 33.3 μM RA. B. LNCaP cells were treated with different dosages (1 to 50 ng/ml) of TNFα for 48 h. C. LNCaP cells were also treated with 20 ng/ml TNFα for different time periods (8 to 48 h). 20 μl of each medium supernatant was subjected to Western blot analysis of midkine expression using rabbit anti-midkine antibodies, horseradish peroxidase-conjungated secondary antibodies and enhanced chemiluminescence reagents. D. Total RNA was extracted from LNCaP cells not treated or treated with 20 ng/ml TNFα, using RNeasy Mini Kit; cDNA was made from total RNA using Superscript™ First-Strand Synthesis System with oligo dT primers; real-time quantitative PCR was done in triplicates with Sybr-Green reagents; results were normalized to GAPDH levels as described in Methods; the data (mean ± standard deviation of three independent experiments) were presented as fold change of midkine mRNA compared to the LNCaP cells without treatment for 8 h, where fold = 2ΔΔCt; solid bar, TNFα treated; open bar, control; * P < 0.05 and **P < 0.01, compared to the corresponding controls. E. PC3 cells were cultured in serum-free medium and treated for 48 h with or without 20 ng/ml TNFα. 20 μl of each medium supernatant was subjected to Western blot analysis of midkine expression.
Figure 3
TNFα induced midkine expression through NF-κB pathway. A. LNCaP cells were not treated or treated for 48 h with increasing dosages of TNFα, without (top blot) or with 18 μM NF-κB inhibitor (bottom blot); 20 μl of each medium supernatant was subjected to Western blot analysis of midkine expression. B. Densitometry of A; the untreated control group was arbitrarily assigned a value of 1; the integrated density values (IDVs) of the protein bands from other groups were divided by that of the control group (i.e., ratio of IDVs) to represent the relative MDK levels; solid line, TNFα only; dotted line, TNFα with 18 μM NF-κB inhibitor. Data presented were average ± standard deviations (error bars) of three independent experiments. C. LNCaP cells were not treated or treated for 48 h with 20 ng/ml TNFα, and without or with increasing dosages of NF-κB inhibitor; 20 μl of each medium supernatant was subjected to Western blot analysis of midkine expression. D. Densitometry of C as described in B. Data presented were average ± standard deviations (error bars) of three independent experiments.
Figure 4
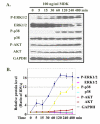
Midkine activated MAP kinase pathways in LNCaP cells. A. The serum-starved LNCaP cells were treated with 100 ng/ml recombinant human MDK for 5 to 480 min; the cells were harvested for protein isolation and Western blot analysis of the indicated proteins; for loading control, the membranes were stripped and probed for GAPDH. B. Densitometry of A; the untreated control group was arbitrarily assigned a value of 1; IDVs of the protein bands from other groups were divided by that of the control group (i.e., ratio of IDVs) to represent the relative individual protein levels over the time course; of note, p38 and GAPDH lines overlapped. Data presented were average ± standard deviations (error bars) of three independent experiments.
Figure 5
Immunohistochemical staining of prostate tissue microarrays. The early stage cancers were from radical prostatectomy specimens derived from patients with clinically localized prostate cancers; while the late stage cancers were derived from transurethral resection specimens of prostate cancers that had advanced beyond the stage treatable by radical prostatectomy; the normal prostate tissues were from the non-tumorous portions of the radical prostatectomy specimens; the prostate tissue microarray slides were stained with 0.6 μg/ml rabbit anti-midkine antibodies using the VECTSTAIN elite ABC Reagent and DAB Substrate Kit according to the manufacturer's protocol and counter-stained with hematoxylin; representative negative (Normal Epithelium and Early Stage Cancer) and strongly positive (Late Stage Cancer) midkine staining are shown; original magnification: × 400.
Similar articles
- Krüppel-like factor 5 promotes apoptosis triggered by tumor necrosis factor α in LNCaP prostate cancer cells via up-regulation of mitogen-activated protein kinase kinase 7.
Shi Q, Gao Y, Xu S, Du C, Li F, Tang XS, Jia J, Wang X, Chang L, He D, Guo P. Shi Q, et al. Urol Oncol. 2016 Feb;34(2):58.e11-8. doi: 10.1016/j.urolonc.2015.09.004. Epub 2015 Oct 21. Urol Oncol. 2016. PMID: 26480897 - Midkine silencing enhances the anti-prostate cancer stem cell activity of the flavone apigenin: cooperation on signaling pathways regulated by ERK, p38, PTEN, PARP, and NF-κB.
Erdogan S, Turkekul K, Dibirdik I, Doganlar ZB, Doganlar O, Bilir A. Erdogan S, et al. Invest New Drugs. 2020 Apr;38(2):246-263. doi: 10.1007/s10637-019-00774-8. Epub 2019 Apr 16. Invest New Drugs. 2020. PMID: 30993586 - Tumor necrosis factor-alpha represses androgen sensitivity in the LNCaP prostate cancer cell line.
Mizokami A, Gotoh A, Yamada H, Keller ET, Matsumoto T. Mizokami A, et al. J Urol. 2000 Sep;164(3 Pt 1):800-5. doi: 10.1097/00005392-200009010-00053. J Urol. 2000. PMID: 10953159 - TNF-alpha/IL-1/NF-kappaB transduction pathway in human cancer prostate.
Royuela M, Rodríguez-Berriguete G, Fraile B, Paniagua R. Royuela M, et al. Histol Histopathol. 2008 Oct;23(10):1279-90. doi: 10.14670/HH-23.1279. Histol Histopathol. 2008. PMID: 18712680 Review. - The midkine family of growth factors: diverse roles in nervous system formation and maintenance.
Winkler C, Yao S. Winkler C, et al. Br J Pharmacol. 2014 Feb;171(4):905-12. doi: 10.1111/bph.12462. Br J Pharmacol. 2014. PMID: 24125182 Free PMC article. Review.
Cited by
- Human macrophages and monocyte-derived dendritic cells stimulate the proliferation of endothelial cells through midkine production.
Said EA, Al-Dughaishi S, Al-Hatmi W, Al-Reesi I, Al-Riyami M, Al-Balushi MS, Al-Bimani A, Al-Busaidi JZ, Al-Khabori M, Al-Kindi S, Procopio FA, Al-Rashdi A, Al-Ansari A, Babiker H, Koh CY, Al-Naamani K, Pantaleo G, Al-Jabri AA. Said EA, et al. PLoS One. 2022 Apr 27;17(4):e0267662. doi: 10.1371/journal.pone.0267662. eCollection 2022. PLoS One. 2022. PMID: 35476724 Free PMC article. - Midkine: a novel prognostic biomarker for cancer.
Jono H, Ando Y. Jono H, et al. Cancers (Basel). 2010 Apr 20;2(2):624-41. doi: 10.3390/cancers2020624. Cancers (Basel). 2010. PMID: 24281085 Free PMC article. - Wnt/β-catenin up-regulates Midkine expression in glioma cells.
Tang SL, Gao YL, Chen XB. Tang SL, et al. Int J Clin Exp Med. 2015 Aug 15;8(8):12644-9. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26550177 Free PMC article. - Role of Midkine in Cancer Drug Resistance: Regulators of Its Expression and Its Molecular Targeting.
Saikia M, Cheung N, Singh AK, Kapoor V. Saikia M, et al. Int J Mol Sci. 2023 May 14;24(10):8739. doi: 10.3390/ijms24108739. Int J Mol Sci. 2023. PMID: 37240085 Free PMC article. Review. - Nasopharynx Battlefield: Cellular Immune Responses Mediated by Midkine in Nasopharyngeal Carcinoma and COVID-19.
Kam NW, Lau CY, Che CM, Lee VH. Kam NW, et al. Cancers (Basel). 2023 Oct 4;15(19):4850. doi: 10.3390/cancers15194850. Cancers (Basel). 2023. PMID: 37835544 Free PMC article. Review.
References
- Kadomatsu K, Tomomura M, Muramatsu T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem Biophys Res Commun. 1988;151:1312–1318. doi: 10.1016/S0006-291X(88)80505-9. - DOI - PubMed
- Tomomura M, Kadomatsu K, Matsubara S, Muramatsu T. A retinoic acid-responsive gene, MK, found in the teratocarcinoma system. Heterogeneity of the transcript and the nature of the translation product. J Biol Chem. 1990;265:10765–10770. - PubMed
- Nakamura E, Kadomatsu K, Yuasa S, Muramatsu H, Mamiya T, Nabeshima T, Fan QW, Ishiguro K, Igakura T, Matsubara S, Kaname T, Horiba M, Saito H, Muramatsu T. Disruption of the midkine gene (Mdk) resulted in altered expression of a calcium binding protein in the hippocampus of infant mice and their abnormal behaviour. Genes Cells. 1998;3:811–822. doi: 10.1046/j.1365-2443.1998.00231.x. - DOI - PubMed
- Zou P, Muramatsu H, Sone M, Hayashi H, Nakashima T, Muramatsu T. Mice doubly deficient in the midkine and pleiotrophin genes exhibit deficits in the expression of beta-tectorin gene and in auditory response. Lab Invest. 2006 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources