Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily - PubMed (original) (raw)
Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily
Jae J Song et al. Cell Signal. 2008 May.
Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has been shown to induce apoptosis through caspase activation in a number of cancer cell lines while displaying minimal or no toxicity on normal cells, suggesting that this protein may hold potential for development as a new cancer therapeutic agent. Moreover, TRAIL can activate mitogen-activated protein kinases (MAPKs) in addition to caspases. However, it has not been clearly understood how MAPKs are activated by TRAIL and the biological significance of their activation. Here we show that TRAIL-induced MAPKs activation is dependent on caspase activation and that mammalian sterile 20-like kinase 1 (Mst1) functions as a mediator between caspase activation and MAPKs activation. Activation of MAPKs (JNK, p38, ERK) is differentially regulated by cleavage size (40 kDa and 36 kDa) of Mst1, which is controlled by caspase-7 and -3.
Figures
Figure 1. TRAIL-induced MAPK activation
DU-145 cells were treated with 200 ng/ml of TRAIL for various times, and the cell lysates were analyzed for the detection of phosphorylated JNK, p38, and ERK as well as PARP and caspase-8. Western blots shown are representative of three independent experiments.
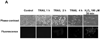
Figure 2. Determination of ROS generation induced by TRAIL
(A) DU-145 cells were treated with TRAIL (200 ng/ml) for indicated times, and hydrogen peroxide generation was measured by incubation with 20 mM fluorescence probe 2’,7’-dichlorofluorescein diacetate (DCF-DA) for 1 h using fluorescence microscope. (B) As an alternative way of measurement of hydrogen peroxide generation, flow cytometry was used. After TRAIL-treated DU-145 cells were incubated with 20 mM fluorescence probe 2’,7’-dichlorofluorescein diacetate (DCF-DA) for 1 h, stained cells were analyzed with FACscan flow cytometer. (C) After catalase-overexpressed DU-145 cells by adenoviral infection expressing catalase (100 moi) or EGFP-overexpressed DU-145 cells by adenoviral infection expressing EGFP as a control (100 moi) were treated with TRAIL (200 ng/ml, 4 h), various MAPKs were examined. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated EGFP-overexpressed cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated catalase-overexpressed cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 2. Determination of ROS generation induced by TRAIL
(A) DU-145 cells were treated with TRAIL (200 ng/ml) for indicated times, and hydrogen peroxide generation was measured by incubation with 20 mM fluorescence probe 2’,7’-dichlorofluorescein diacetate (DCF-DA) for 1 h using fluorescence microscope. (B) As an alternative way of measurement of hydrogen peroxide generation, flow cytometry was used. After TRAIL-treated DU-145 cells were incubated with 20 mM fluorescence probe 2’,7’-dichlorofluorescein diacetate (DCF-DA) for 1 h, stained cells were analyzed with FACscan flow cytometer. (C) After catalase-overexpressed DU-145 cells by adenoviral infection expressing catalase (100 moi) or EGFP-overexpressed DU-145 cells by adenoviral infection expressing EGFP as a control (100 moi) were treated with TRAIL (200 ng/ml, 4 h), various MAPKs were examined. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated EGFP-overexpressed cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated catalase-overexpressed cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 2. Determination of ROS generation induced by TRAIL
(A) DU-145 cells were treated with TRAIL (200 ng/ml) for indicated times, and hydrogen peroxide generation was measured by incubation with 20 mM fluorescence probe 2’,7’-dichlorofluorescein diacetate (DCF-DA) for 1 h using fluorescence microscope. (B) As an alternative way of measurement of hydrogen peroxide generation, flow cytometry was used. After TRAIL-treated DU-145 cells were incubated with 20 mM fluorescence probe 2’,7’-dichlorofluorescein diacetate (DCF-DA) for 1 h, stained cells were analyzed with FACscan flow cytometer. (C) After catalase-overexpressed DU-145 cells by adenoviral infection expressing catalase (100 moi) or EGFP-overexpressed DU-145 cells by adenoviral infection expressing EGFP as a control (100 moi) were treated with TRAIL (200 ng/ml, 4 h), various MAPKs were examined. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated EGFP-overexpressed cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated catalase-overexpressed cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 3. Involvement of caspase-8 during TRAIL-induced MAPKs activation in DU-145
(A) After caspase-8 inhibitor (Z-IETD-FMK 20 µM) was pretreated for 30 min, followed by TRAIL treatment (200 ng/ml) for 4 h, various MAPKs were examined. Left panel: Western blot analysis. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated DU-145 cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated DU-145 cells with caspase-8 inhibitor pretreatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) After caspase-8 was downregulated by siRNA of caspase-8, TRAIL was treated for the examination of various MAPKs. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated si scrambled RNA-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated si caspase-8 RNA-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 3. Involvement of caspase-8 during TRAIL-induced MAPKs activation in DU-145
(A) After caspase-8 inhibitor (Z-IETD-FMK 20 µM) was pretreated for 30 min, followed by TRAIL treatment (200 ng/ml) for 4 h, various MAPKs were examined. Left panel: Western blot analysis. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated DU-145 cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated DU-145 cells with caspase-8 inhibitor pretreatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) After caspase-8 was downregulated by siRNA of caspase-8, TRAIL was treated for the examination of various MAPKs. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated si scrambled RNA-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated si caspase-8 RNA-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 4. Involvement of caspase-7 or -3 during TRAIL-induced MAPKs activation in DU-145
(A) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after caspase-7 was downregulated by siRNA of caspase-7. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated si scrambled RNA-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated si caspase-7 RNA-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after caspase-3 was downregulated by siRNA of caspase-3. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled RNA-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si caspase-3 RNA-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 4. Involvement of caspase-7 or -3 during TRAIL-induced MAPKs activation in DU-145
(A) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after caspase-7 was downregulated by siRNA of caspase-7. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated si scrambled RNA-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated si caspase-7 RNA-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after caspase-3 was downregulated by siRNA of caspase-3. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled RNA-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si caspase-3 RNA-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
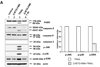
Figure 5. Involvement of caspase-8, -7 and -3 during TRAIL-induced MAPKs activation in MCF-7
(A) After caspase-8 inhibitor (Z-IETD-FMK 20 µM) was pretreated for 30 min, MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined in MCF-7 cells that lack caspase-3. Left panel: Western blot analysis. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7 cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7 cells with caspase-8 inhibitor pretreatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after caspase-7 was downregulated by siRNA of caspase-7 in caspase-3-deficient MCF-7 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated si scrambled RNA-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated si caspase-7 RNA-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (C) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined in MCF-7 cells overexpressing caspase-3. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7/pcDNA3 cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7/caspase-3 cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) After caspase-3 transfection in dose-dependent manner, MAPKs phosphorylation was examined after TRAIL treatment (200 ng/ml, 4 h) in MCF-7 cells overexpressing caspase-3. Upper panel: Western blot analysis, C, control. Lower panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated cells with no caspase-3 transfection was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated cells with different amounts of caspase-3 transfection was compared to this. Note the difference in vertical scale of the three subpanels. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 5. Involvement of caspase-8, -7 and -3 during TRAIL-induced MAPKs activation in MCF-7
(A) After caspase-8 inhibitor (Z-IETD-FMK 20 µM) was pretreated for 30 min, MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined in MCF-7 cells that lack caspase-3. Left panel: Western blot analysis. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7 cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7 cells with caspase-8 inhibitor pretreatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after caspase-7 was downregulated by siRNA of caspase-7 in caspase-3-deficient MCF-7 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated si scrambled RNA-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated si caspase-7 RNA-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (C) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined in MCF-7 cells overexpressing caspase-3. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7/pcDNA3 cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7/caspase-3 cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) After caspase-3 transfection in dose-dependent manner, MAPKs phosphorylation was examined after TRAIL treatment (200 ng/ml, 4 h) in MCF-7 cells overexpressing caspase-3. Upper panel: Western blot analysis, C, control. Lower panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated cells with no caspase-3 transfection was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated cells with different amounts of caspase-3 transfection was compared to this. Note the difference in vertical scale of the three subpanels. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 5. Involvement of caspase-8, -7 and -3 during TRAIL-induced MAPKs activation in MCF-7
(A) After caspase-8 inhibitor (Z-IETD-FMK 20 µM) was pretreated for 30 min, MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined in MCF-7 cells that lack caspase-3. Left panel: Western blot analysis. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7 cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7 cells with caspase-8 inhibitor pretreatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after caspase-7 was downregulated by siRNA of caspase-7 in caspase-3-deficient MCF-7 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated si scrambled RNA-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated si caspase-7 RNA-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (C) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined in MCF-7 cells overexpressing caspase-3. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7/pcDNA3 cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7/caspase-3 cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) After caspase-3 transfection in dose-dependent manner, MAPKs phosphorylation was examined after TRAIL treatment (200 ng/ml, 4 h) in MCF-7 cells overexpressing caspase-3. Upper panel: Western blot analysis, C, control. Lower panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated cells with no caspase-3 transfection was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated cells with different amounts of caspase-3 transfection was compared to this. Note the difference in vertical scale of the three subpanels. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 5. Involvement of caspase-8, -7 and -3 during TRAIL-induced MAPKs activation in MCF-7
(A) After caspase-8 inhibitor (Z-IETD-FMK 20 µM) was pretreated for 30 min, MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined in MCF-7 cells that lack caspase-3. Left panel: Western blot analysis. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7 cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7 cells with caspase-8 inhibitor pretreatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after caspase-7 was downregulated by siRNA of caspase-7 in caspase-3-deficient MCF-7 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated si scrambled RNA-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated si caspase-7 RNA-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (C) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined in MCF-7 cells overexpressing caspase-3. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7/pcDNA3 cells was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin protein in TRAIL-treated MCF-7/caspase-3 cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) After caspase-3 transfection in dose-dependent manner, MAPKs phosphorylation was examined after TRAIL treatment (200 ng/ml, 4 h) in MCF-7 cells overexpressing caspase-3. Upper panel: Western blot analysis, C, control. Lower panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated cells with no caspase-3 transfection was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated cells with different amounts of caspase-3 transfection was compared to this. Note the difference in vertical scale of the three subpanels. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 6. Mst1 as a mediator of caspase activation to MAPKs phosphorylation during TRAIL treatment
(A) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after transfection of control vector (pSilencer) or pSilencer-siMst1 into DU-145 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si caspase-7 plasmid-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) Immunoblots were made of Mst1 expression in control vector transfected (pSilencer) or pSilencer-siMst1 stably transfected single cell clones from DU-145 cells. Lysates containing equal amounts of proteins (20 µg) were separated by SDS-PAGE and immunoblotted with anti-Mst1 antibody. (C) Control plasmid (pSilencer) or pSilencer-siMst1 stably transfected cells (clone #6) or a pool of selected clones (#6, #7, and #8) was treated with TRAIL (200 ng/ml) for 4 h, and phosphorylation of MAPKs was examined. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected stable cell clone or clone pool was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si Mst1 plasmid-transfected stable clone or pool was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) Mst1 cleavage was examined in the presence of caspase-8 inhibitor (Z-IETD-FMK 20 µM, 30 min) pretreatment before TRAIL treatment (200 ng/ml, 2 h or 4 h) in DU-145 cells. Upper panel: Western blot analysis. Lower panel: The ratio of the uncleaved caspase-8 or Mst1 to corresponding actin at 0 h was set equal to 1 (not shown), and the ratio of the cleaved caspase-8 or cleaved Mst1 to corresponding actin at 2 h and 4 h of TRAIL treatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (E, F) Shift of cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 0–4 h) was examined in by Western blot analysis in DU-145 cells or MCF-7 cells, respectively. Lower panel: The ratio of the 40 kDa cleaved Mst1 to corresponding actin at 2 h was set equal to 1, and the ratio of the 40 kDa cleaved Mst1 to corresponding actin at 3 h and 4 h was compared to this, then again the 40 kDa cleaved Mst1 at 2 h was set equal to 1, and compared with 36 kDa cleaved Mst1 for hours 2, 3 and 4. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (G) Involvement of caspase-7 with the cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 2 h) was examined in DU-145 cells after caspase-7 was downregulated by siRNA of caspase-7. Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells was compared to the ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 6. Mst1 as a mediator of caspase activation to MAPKs phosphorylation during TRAIL treatment
(A) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after transfection of control vector (pSilencer) or pSilencer-siMst1 into DU-145 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si caspase-7 plasmid-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) Immunoblots were made of Mst1 expression in control vector transfected (pSilencer) or pSilencer-siMst1 stably transfected single cell clones from DU-145 cells. Lysates containing equal amounts of proteins (20 µg) were separated by SDS-PAGE and immunoblotted with anti-Mst1 antibody. (C) Control plasmid (pSilencer) or pSilencer-siMst1 stably transfected cells (clone #6) or a pool of selected clones (#6, #7, and #8) was treated with TRAIL (200 ng/ml) for 4 h, and phosphorylation of MAPKs was examined. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected stable cell clone or clone pool was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si Mst1 plasmid-transfected stable clone or pool was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) Mst1 cleavage was examined in the presence of caspase-8 inhibitor (Z-IETD-FMK 20 µM, 30 min) pretreatment before TRAIL treatment (200 ng/ml, 2 h or 4 h) in DU-145 cells. Upper panel: Western blot analysis. Lower panel: The ratio of the uncleaved caspase-8 or Mst1 to corresponding actin at 0 h was set equal to 1 (not shown), and the ratio of the cleaved caspase-8 or cleaved Mst1 to corresponding actin at 2 h and 4 h of TRAIL treatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (E, F) Shift of cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 0–4 h) was examined in by Western blot analysis in DU-145 cells or MCF-7 cells, respectively. Lower panel: The ratio of the 40 kDa cleaved Mst1 to corresponding actin at 2 h was set equal to 1, and the ratio of the 40 kDa cleaved Mst1 to corresponding actin at 3 h and 4 h was compared to this, then again the 40 kDa cleaved Mst1 at 2 h was set equal to 1, and compared with 36 kDa cleaved Mst1 for hours 2, 3 and 4. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (G) Involvement of caspase-7 with the cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 2 h) was examined in DU-145 cells after caspase-7 was downregulated by siRNA of caspase-7. Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells was compared to the ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 6. Mst1 as a mediator of caspase activation to MAPKs phosphorylation during TRAIL treatment
(A) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after transfection of control vector (pSilencer) or pSilencer-siMst1 into DU-145 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si caspase-7 plasmid-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) Immunoblots were made of Mst1 expression in control vector transfected (pSilencer) or pSilencer-siMst1 stably transfected single cell clones from DU-145 cells. Lysates containing equal amounts of proteins (20 µg) were separated by SDS-PAGE and immunoblotted with anti-Mst1 antibody. (C) Control plasmid (pSilencer) or pSilencer-siMst1 stably transfected cells (clone #6) or a pool of selected clones (#6, #7, and #8) was treated with TRAIL (200 ng/ml) for 4 h, and phosphorylation of MAPKs was examined. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected stable cell clone or clone pool was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si Mst1 plasmid-transfected stable clone or pool was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) Mst1 cleavage was examined in the presence of caspase-8 inhibitor (Z-IETD-FMK 20 µM, 30 min) pretreatment before TRAIL treatment (200 ng/ml, 2 h or 4 h) in DU-145 cells. Upper panel: Western blot analysis. Lower panel: The ratio of the uncleaved caspase-8 or Mst1 to corresponding actin at 0 h was set equal to 1 (not shown), and the ratio of the cleaved caspase-8 or cleaved Mst1 to corresponding actin at 2 h and 4 h of TRAIL treatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (E, F) Shift of cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 0–4 h) was examined in by Western blot analysis in DU-145 cells or MCF-7 cells, respectively. Lower panel: The ratio of the 40 kDa cleaved Mst1 to corresponding actin at 2 h was set equal to 1, and the ratio of the 40 kDa cleaved Mst1 to corresponding actin at 3 h and 4 h was compared to this, then again the 40 kDa cleaved Mst1 at 2 h was set equal to 1, and compared with 36 kDa cleaved Mst1 for hours 2, 3 and 4. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (G) Involvement of caspase-7 with the cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 2 h) was examined in DU-145 cells after caspase-7 was downregulated by siRNA of caspase-7. Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells was compared to the ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 6. Mst1 as a mediator of caspase activation to MAPKs phosphorylation during TRAIL treatment
(A) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after transfection of control vector (pSilencer) or pSilencer-siMst1 into DU-145 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si caspase-7 plasmid-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) Immunoblots were made of Mst1 expression in control vector transfected (pSilencer) or pSilencer-siMst1 stably transfected single cell clones from DU-145 cells. Lysates containing equal amounts of proteins (20 µg) were separated by SDS-PAGE and immunoblotted with anti-Mst1 antibody. (C) Control plasmid (pSilencer) or pSilencer-siMst1 stably transfected cells (clone #6) or a pool of selected clones (#6, #7, and #8) was treated with TRAIL (200 ng/ml) for 4 h, and phosphorylation of MAPKs was examined. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected stable cell clone or clone pool was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si Mst1 plasmid-transfected stable clone or pool was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) Mst1 cleavage was examined in the presence of caspase-8 inhibitor (Z-IETD-FMK 20 µM, 30 min) pretreatment before TRAIL treatment (200 ng/ml, 2 h or 4 h) in DU-145 cells. Upper panel: Western blot analysis. Lower panel: The ratio of the uncleaved caspase-8 or Mst1 to corresponding actin at 0 h was set equal to 1 (not shown), and the ratio of the cleaved caspase-8 or cleaved Mst1 to corresponding actin at 2 h and 4 h of TRAIL treatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (E, F) Shift of cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 0–4 h) was examined in by Western blot analysis in DU-145 cells or MCF-7 cells, respectively. Lower panel: The ratio of the 40 kDa cleaved Mst1 to corresponding actin at 2 h was set equal to 1, and the ratio of the 40 kDa cleaved Mst1 to corresponding actin at 3 h and 4 h was compared to this, then again the 40 kDa cleaved Mst1 at 2 h was set equal to 1, and compared with 36 kDa cleaved Mst1 for hours 2, 3 and 4. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (G) Involvement of caspase-7 with the cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 2 h) was examined in DU-145 cells after caspase-7 was downregulated by siRNA of caspase-7. Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells was compared to the ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 6. Mst1 as a mediator of caspase activation to MAPKs phosphorylation during TRAIL treatment
(A) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after transfection of control vector (pSilencer) or pSilencer-siMst1 into DU-145 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si caspase-7 plasmid-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) Immunoblots were made of Mst1 expression in control vector transfected (pSilencer) or pSilencer-siMst1 stably transfected single cell clones from DU-145 cells. Lysates containing equal amounts of proteins (20 µg) were separated by SDS-PAGE and immunoblotted with anti-Mst1 antibody. (C) Control plasmid (pSilencer) or pSilencer-siMst1 stably transfected cells (clone #6) or a pool of selected clones (#6, #7, and #8) was treated with TRAIL (200 ng/ml) for 4 h, and phosphorylation of MAPKs was examined. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected stable cell clone or clone pool was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si Mst1 plasmid-transfected stable clone or pool was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) Mst1 cleavage was examined in the presence of caspase-8 inhibitor (Z-IETD-FMK 20 µM, 30 min) pretreatment before TRAIL treatment (200 ng/ml, 2 h or 4 h) in DU-145 cells. Upper panel: Western blot analysis. Lower panel: The ratio of the uncleaved caspase-8 or Mst1 to corresponding actin at 0 h was set equal to 1 (not shown), and the ratio of the cleaved caspase-8 or cleaved Mst1 to corresponding actin at 2 h and 4 h of TRAIL treatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (E, F) Shift of cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 0–4 h) was examined in by Western blot analysis in DU-145 cells or MCF-7 cells, respectively. Lower panel: The ratio of the 40 kDa cleaved Mst1 to corresponding actin at 2 h was set equal to 1, and the ratio of the 40 kDa cleaved Mst1 to corresponding actin at 3 h and 4 h was compared to this, then again the 40 kDa cleaved Mst1 at 2 h was set equal to 1, and compared with 36 kDa cleaved Mst1 for hours 2, 3 and 4. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (G) Involvement of caspase-7 with the cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 2 h) was examined in DU-145 cells after caspase-7 was downregulated by siRNA of caspase-7. Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells was compared to the ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 6. Mst1 as a mediator of caspase activation to MAPKs phosphorylation during TRAIL treatment
(A) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after transfection of control vector (pSilencer) or pSilencer-siMst1 into DU-145 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si caspase-7 plasmid-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) Immunoblots were made of Mst1 expression in control vector transfected (pSilencer) or pSilencer-siMst1 stably transfected single cell clones from DU-145 cells. Lysates containing equal amounts of proteins (20 µg) were separated by SDS-PAGE and immunoblotted with anti-Mst1 antibody. (C) Control plasmid (pSilencer) or pSilencer-siMst1 stably transfected cells (clone #6) or a pool of selected clones (#6, #7, and #8) was treated with TRAIL (200 ng/ml) for 4 h, and phosphorylation of MAPKs was examined. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected stable cell clone or clone pool was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si Mst1 plasmid-transfected stable clone or pool was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) Mst1 cleavage was examined in the presence of caspase-8 inhibitor (Z-IETD-FMK 20 µM, 30 min) pretreatment before TRAIL treatment (200 ng/ml, 2 h or 4 h) in DU-145 cells. Upper panel: Western blot analysis. Lower panel: The ratio of the uncleaved caspase-8 or Mst1 to corresponding actin at 0 h was set equal to 1 (not shown), and the ratio of the cleaved caspase-8 or cleaved Mst1 to corresponding actin at 2 h and 4 h of TRAIL treatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (E, F) Shift of cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 0–4 h) was examined in by Western blot analysis in DU-145 cells or MCF-7 cells, respectively. Lower panel: The ratio of the 40 kDa cleaved Mst1 to corresponding actin at 2 h was set equal to 1, and the ratio of the 40 kDa cleaved Mst1 to corresponding actin at 3 h and 4 h was compared to this, then again the 40 kDa cleaved Mst1 at 2 h was set equal to 1, and compared with 36 kDa cleaved Mst1 for hours 2, 3 and 4. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (G) Involvement of caspase-7 with the cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 2 h) was examined in DU-145 cells after caspase-7 was downregulated by siRNA of caspase-7. Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells was compared to the ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 6. Mst1 as a mediator of caspase activation to MAPKs phosphorylation during TRAIL treatment
(A) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after transfection of control vector (pSilencer) or pSilencer-siMst1 into DU-145 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si caspase-7 plasmid-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) Immunoblots were made of Mst1 expression in control vector transfected (pSilencer) or pSilencer-siMst1 stably transfected single cell clones from DU-145 cells. Lysates containing equal amounts of proteins (20 µg) were separated by SDS-PAGE and immunoblotted with anti-Mst1 antibody. (C) Control plasmid (pSilencer) or pSilencer-siMst1 stably transfected cells (clone #6) or a pool of selected clones (#6, #7, and #8) was treated with TRAIL (200 ng/ml) for 4 h, and phosphorylation of MAPKs was examined. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected stable cell clone or clone pool was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si Mst1 plasmid-transfected stable clone or pool was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) Mst1 cleavage was examined in the presence of caspase-8 inhibitor (Z-IETD-FMK 20 µM, 30 min) pretreatment before TRAIL treatment (200 ng/ml, 2 h or 4 h) in DU-145 cells. Upper panel: Western blot analysis. Lower panel: The ratio of the uncleaved caspase-8 or Mst1 to corresponding actin at 0 h was set equal to 1 (not shown), and the ratio of the cleaved caspase-8 or cleaved Mst1 to corresponding actin at 2 h and 4 h of TRAIL treatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (E, F) Shift of cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 0–4 h) was examined in by Western blot analysis in DU-145 cells or MCF-7 cells, respectively. Lower panel: The ratio of the 40 kDa cleaved Mst1 to corresponding actin at 2 h was set equal to 1, and the ratio of the 40 kDa cleaved Mst1 to corresponding actin at 3 h and 4 h was compared to this, then again the 40 kDa cleaved Mst1 at 2 h was set equal to 1, and compared with 36 kDa cleaved Mst1 for hours 2, 3 and 4. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (G) Involvement of caspase-7 with the cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 2 h) was examined in DU-145 cells after caspase-7 was downregulated by siRNA of caspase-7. Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells was compared to the ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 6. Mst1 as a mediator of caspase activation to MAPKs phosphorylation during TRAIL treatment
(A) MAPKs phosphorylation induced by TRAIL treatment (200 ng/ml, 4 h) was examined after transfection of control vector (pSilencer) or pSilencer-siMst1 into DU-145 cells. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected cells was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si caspase-7 plasmid-transfected cells was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) Immunoblots were made of Mst1 expression in control vector transfected (pSilencer) or pSilencer-siMst1 stably transfected single cell clones from DU-145 cells. Lysates containing equal amounts of proteins (20 µg) were separated by SDS-PAGE and immunoblotted with anti-Mst1 antibody. (C) Control plasmid (pSilencer) or pSilencer-siMst1 stably transfected cells (clone #6) or a pool of selected clones (#6, #7, and #8) was treated with TRAIL (200 ng/ml) for 4 h, and phosphorylation of MAPKs was examined. Left panel: Western blot analysis, C, control; T, TRAIL. Right panel: The ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si scrambled plasmid-transfected stable cell clone or clone pool was set equal to 1, and the ratio of the phosphorylated MAPKs to total protein of corresponding MAPKs in TRAIL-treated si Mst1 plasmid-transfected stable clone or pool was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (D) Mst1 cleavage was examined in the presence of caspase-8 inhibitor (Z-IETD-FMK 20 µM, 30 min) pretreatment before TRAIL treatment (200 ng/ml, 2 h or 4 h) in DU-145 cells. Upper panel: Western blot analysis. Lower panel: The ratio of the uncleaved caspase-8 or Mst1 to corresponding actin at 0 h was set equal to 1 (not shown), and the ratio of the cleaved caspase-8 or cleaved Mst1 to corresponding actin at 2 h and 4 h of TRAIL treatment was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (E, F) Shift of cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 0–4 h) was examined in by Western blot analysis in DU-145 cells or MCF-7 cells, respectively. Lower panel: The ratio of the 40 kDa cleaved Mst1 to corresponding actin at 2 h was set equal to 1, and the ratio of the 40 kDa cleaved Mst1 to corresponding actin at 3 h and 4 h was compared to this, then again the 40 kDa cleaved Mst1 at 2 h was set equal to 1, and compared with 36 kDa cleaved Mst1 for hours 2, 3 and 4. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (G) Involvement of caspase-7 with the cleaved forms of Mst1 during TRAIL treatment (200 ng/ml, 2 h) was examined in DU-145 cells after caspase-7 was downregulated by siRNA of caspase-7. Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si scrambled RNA-transfected cells was compared to the ratio of the 20 kDa cleaved caspase-7 (or 40 kDa cleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells to 35 kDa uncleaved caspase-7 (or 59 kDa uncleaved Mst1) in TRAIL-treated si caspase-7 RNA-transfected cells. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
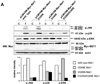
Figure 7. Differential MAPKs regulation by Mst1 cleavage size in DU-145
(A) MAPKs phosphorylation was examined after transfection of various mutants of Mst1 in which D326, D349, and both D326 and D349 were mutated. Each construct was transfected into Mst1-knockdown selected cell clone (#6), then after 48 h of transfection, phosphorylation of various MAPKs was examined after the cells were treated with TRAIL (200 ng/ml, 4 h). Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated cells was set equal to 1 in wild-type, and the ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated cells with different Mst1 deletions was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) After construction of several deletion mutants of Myc-tagged Mst1 (Myc-tagged 1–349 amino acids of Mst1, Myc-tagged 1–349 amino acids with D326N of Mst1, and Myc-tagged 1–326 amino acids of Mst1), each deletion mutant was transfected into Mst1-knockdown selected cell clone (#6). After 48 h of transfection, phosphorylation of various MAPKs was examined after the cells were treated with TRAIL (200 ng/ml, 4 h). Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated cells in wild-type was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated cells with different Myc-tagged Mst1 deletions was compared to this. Note the difference in vertical scale of the three sub-panels. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 7. Differential MAPKs regulation by Mst1 cleavage size in DU-145
(A) MAPKs phosphorylation was examined after transfection of various mutants of Mst1 in which D326, D349, and both D326 and D349 were mutated. Each construct was transfected into Mst1-knockdown selected cell clone (#6), then after 48 h of transfection, phosphorylation of various MAPKs was examined after the cells were treated with TRAIL (200 ng/ml, 4 h). Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated cells was set equal to 1 in wild-type, and the ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated cells with different Mst1 deletions was compared to this. Data are expressed as mean ± SE of the densitometry data from three independent experiments. (B) After construction of several deletion mutants of Myc-tagged Mst1 (Myc-tagged 1–349 amino acids of Mst1, Myc-tagged 1–349 amino acids with D326N of Mst1, and Myc-tagged 1–326 amino acids of Mst1), each deletion mutant was transfected into Mst1-knockdown selected cell clone (#6). After 48 h of transfection, phosphorylation of various MAPKs was examined after the cells were treated with TRAIL (200 ng/ml, 4 h). Upper panel: Western blot analysis, C, control; T, TRAIL. Lower panel: The ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated cells in wild-type was set equal to 1, and the ratio of the phosphorylated MAPKs to corresponding actin in TRAIL-treated cells with different Myc-tagged Mst1 deletions was compared to this. Note the difference in vertical scale of the three sub-panels. Data are expressed as mean ± SE of the densitometry data from three independent experiments.
Figure 8. Schematic diagram of Mst1 as a mediator between TRAIL-induced caspase activation and MAPK activation
Similar articles
- Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced mitochondrial pathway to apoptosis and caspase activation is potentiated by phospholipid scramblase-3.
Ndebele K, Gona P, Jin TG, Benhaga N, Chalah A, Degli-Esposti M, Khosravi-Far R. Ndebele K, et al. Apoptosis. 2008 Jul;13(7):845-56. doi: 10.1007/s10495-008-0219-4. Apoptosis. 2008. PMID: 18491232 Free PMC article. - MAPK p38 and JNK have opposing activities on TRAIL-induced apoptosis activation in NSCLC H460 cells that involves RIP1 and caspase-8 and is mediated by Mcl-1.
Azijli K, Yuvaraj S, van Roosmalen I, Flach K, Giovannetti E, Peters GJ, de Jong S, Kruyt FA. Azijli K, et al. Apoptosis. 2013 Jul;18(7):851-60. doi: 10.1007/s10495-013-0829-3. Apoptosis. 2013. PMID: 23456625 - MEKK1/MEKK4 are responsible for TRAIL-induced JNK/p38 phosphorylation.
Sun BK, Kim JH, Nguyen HN, Oh S, Kim SY, Choi S, Choi HJ, Lee YJ, Song JJ. Sun BK, et al. Oncol Rep. 2011 Feb;25(2):537-44. doi: 10.3892/or.2010.1079. Epub 2010 Dec 7. Oncol Rep. 2011. PMID: 21152872 Free PMC article. - The MEK-ERK-MST1 Axis Potentiates the Activation of the Extrinsic Apoptotic Pathway during GDC-0941 Treatment in Jurkat T Cells.
Nováková J, Talacko P, Novák P, Vališ K. Nováková J, et al. Cells. 2019 Feb 21;8(2):191. doi: 10.3390/cells8020191. Cells. 2019. PMID: 30795621 Free PMC article.
Cited by
- TRAIL and interferon-alpha act synergistically to induce renal cell carcinoma apoptosis.
Clark PE, Polosukhina DA, Gyabaah K, Moses HL, Thorburn A, Zent R. Clark PE, et al. J Urol. 2010 Sep;184(3):1166-74. doi: 10.1016/j.juro.2010.04.064. Epub 2010 Jul 21. J Urol. 2010. PMID: 20663526 Free PMC article. - Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell!
Fan Y, Bergmann A. Fan Y, et al. Trends Cell Biol. 2008 Oct;18(10):467-73. doi: 10.1016/j.tcb.2008.08.001. Epub 2008 Sep 4. Trends Cell Biol. 2008. PMID: 18774295 Free PMC article. Review. - Potential prognostic significance of decreased serum levels of TRAIL after acute myocardial infarction.
Secchiero P, Corallini F, Ceconi C, Parrinello G, Volpato S, Ferrari R, Zauli G. Secchiero P, et al. PLoS One. 2009;4(2):e4442. doi: 10.1371/journal.pone.0004442. Epub 2009 Feb 16. PLoS One. 2009. PMID: 19221598 Free PMC article. - The "O" class: crafting clinical care with FoxO transcription factors.
Maiese K, Chong ZZ, Hou J, Shang YC. Maiese K, et al. Adv Exp Med Biol. 2009;665:242-60. doi: 10.1007/978-1-4419-1599-3_18. Adv Exp Med Biol. 2009. PMID: 20429429 Free PMC article. Review. - Reactive oxygen species: a double-edged sword in oncogenesis.
Pan JS, Hong MZ, Ren JL. Pan JS, et al. World J Gastroenterol. 2009 Apr 14;15(14):1702-7. doi: 10.3748/wjg.15.1702. World J Gastroenterol. 2009. PMID: 19360913 Free PMC article. Review.
References
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. J. Biol. Chem. 1996;271:12687. - PubMed
- Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. Immunity. 1997b;7:813. - PubMed
- Lu Y, Cai Z, Xiao G, Keller ET, Mizokami A, Yao Z, Roodman GD, Zhang J. Cancer Res. 2007;67:3646. - PubMed
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. Science. 1997a;277:815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA96989/CA/NCI NIH HHS/United States
- R01 CA096989/CA/NCI NIH HHS/United States
- CA121395/CA/NCI NIH HHS/United States
- R01 CA096989-01A2/CA/NCI NIH HHS/United States
- R03 CA121395-01/CA/NCI NIH HHS/United States
- R01 CA095191-01A2/CA/NCI NIH HHS/United States
- CA95191/CA/NCI NIH HHS/United States
- R03 CA121395/CA/NCI NIH HHS/United States
- R01 CA095191/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous