Collagen 11a1 is indirectly activated by lymphocyte enhancer-binding factor 1 (Lef1) and negatively regulates osteoblast maturation - PubMed (original) (raw)
Collagen 11a1 is indirectly activated by lymphocyte enhancer-binding factor 1 (Lef1) and negatively regulates osteoblast maturation
Rachel A Kahler et al. Matrix Biol. 2008 May.
Abstract
Alpha 1 (XI) collagen (Col11a1) is essential for normal skeletal development. Mutations in Col11a1 cause Marshall and Stickler syndromes, both of which are characterized by craniofacial abnormalities, nearsightedness and hearing deficiencies. Despite its link to human diseases, few studies have described factors that control Col11a1 transcription. We previously identified Col11a1 as a differentially expressed gene in Lef1-suppressed MC3T3 preosteoblasts. Here we report that Lef1 activates the Col11a1 promoter. This activation is dependent upon the DNA binding domain of Lef1, but does not require the beta-catenin interaction domain, suggesting that it is not responsive to Wnt signals. Targeted suppression of Col11a1 with an antisense morpholino accelerated osteoblastic differentiation and mineralization in C2C12 cells, similar to what was observed in Lef1-suppressed MC3T3 cells. Moreover incubation with a purified Col11a1 N-terminal fragment, V1B, prevented alkaline phosphatase expression in MC3T3 and C2C12 cells. These results suggest that Lef1 is an activator of the Col11a1 promoter and that Col11a1 suppresses terminal osteoblast differentiation.
Figures
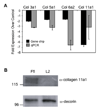
Figure 1. Col11a1 expression is reduced in Lef1-deficient cells
A. The relative change in expression of collagen 11a1 in Lef1-suppressed MC3T3 cells (L2) as compared to control cells expressing a short hairpin RNA against firefly luciferase (Ffl). Black bars depict fold reduction of expression ± SD as measured by gene chip analysis with a p-value ≤ 0.05. Grey bars depict fold reduction of expression ± SD as measured by quantitative RT-PCR. B. Immunoblot analysis of protein precipitated from L2 or Ffl cell-conditioned media. The upper panel is blotted with α-collagen 11a1 antibody. The lower panel is blotted with α-decorin antibody as a protein loading control.
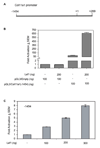
Figure 2. Lef1 activates the Col11a1 promoter
A. This schematic depicts the
Col11a1
promoter used in this study. pCol11a1(−1454)-luc encompasses bases −1454 to +269. B. C2C12 cells were transfected with pGL3 or pGL3-Col11a1(−1454)-luc, pRL-null and 200 ng of either pcDNA (−) or pCMV5B-Lef1-HA. Transcription activation is shown as fold luciferase activity over control ± SEM and corrected for transfection efficiency relative to renilla expression. C. C2C12 cells were transfected with pGL3-Col11a1(−1454)-luc, pRL-null and the indicated concentrations of pCMV5B-Lef1-HA. Data were analyzed as in B.
Figure 3. The HMG domain of Lef1 is required for activation of Col11a1 promoter
A. This schematic depicts the Lef1, Lef1ΔHMG, and Lef1Δβ-cat expression constructs used in these experiments. β-cat BD, β-catenin binding domain; TLE BD, TLE binding domain; HMG,,high mobility group domain. B. C2C12 cells were transfected with pCol11a1(−1454)-luc, pRL-null and 200 ng of either pcDNA3 (−), pCMV5-Lef1-HA (Lef1), or pCMV5-Lef1ΔHMG-HA (ΔHMG) as indicated. Transcription is measured as fold luciferase activity over control ± SEM. C. C2C12 cells were transfected with pCol11(−1454)-luc, pRL-null, 200 ng pCMV5B-Lef1-HA and either 200 ng pcDNA3 (−) or the indicated amount of Lef1Δβ-cat. Transcription is measured as fold luciferase activity over control ± SEM.
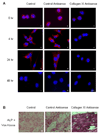
Figure 4. Col11a1 antisense morpholinos induce increased expression of osteoblast markers
A. C2C12 cells were treated with either no (left column), control (center column) or Col11a1 antisense morpholinos (right column) and immunofluorescently stained with an anti-Col11a1 primary antibody and a TRITC-labeled secondary antibody. C2C12 cells were induced to differentiate into the osteoblastic lineage by treatment with BMP-2 for the indicated length of time. B. C2C12 cells were treated either with no (A), control (B) or Col11a1 antisense morpholinos (C) and stained for alkaline phosphatase activity (red) and mineralization (von Kossa staining, black) after 14 days treatment with BMP-2.
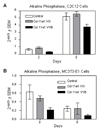
Figure 5. Alkaline phosphatase expression is inhibited during osteoblast differentiation by an N-terminal Col11a1 fragment, V1B
C2C12 cells were differentiated in osteogenic medium supplemented with BMP2 for five days (A) and MC3T3-E1 cells were differentiated in osteogenic medium for nine days (B) in the presence of vehicle alone, Col11a1 V0 fragment, or Col11a1 V1B fragment. RNA was collected at the indicated time points and quantitative PCR was performed using alkaline phosphatase and actin specific primers. Alkaline phosphatase values were normalized to actin.
Similar articles
- Collagen Type XI Alpha 1 (COL11A1): A Novel Biomarker and a Key Player in Cancer.
Nallanthighal S, Heiserman JP, Cheon DJ. Nallanthighal S, et al. Cancers (Basel). 2021 Feb 24;13(5):935. doi: 10.3390/cancers13050935. Cancers (Basel). 2021. PMID: 33668097 Free PMC article. Review. - Lymphocyte enhancer-binding factor 1 (Lef1) inhibits terminal differentiation of osteoblasts.
Kahler RA, Galindo M, Lian J, Stein GS, van Wijnen AJ, Westendorf JJ. Kahler RA, et al. J Cell Biochem. 2006 Apr 1;97(5):969-83. doi: 10.1002/jcb.20702. J Cell Biochem. 2006. PMID: 16267835 - Lef1DeltaN binds beta-catenin and increases osteoblast activity and trabecular bone mass.
Hoeppner LH, Secreto FJ, Razidlo DF, Whitney TJ, Westendorf JJ. Hoeppner LH, et al. J Biol Chem. 2011 Apr 1;286(13):10950-9. doi: 10.1074/jbc.M110.165100. Epub 2011 Jan 26. J Biol Chem. 2011. PMID: 21270130 Free PMC article. - Differentiation-inducing factor-1 alters canonical Wnt signaling and suppresses alkaline phosphatase expression in osteoblast-like cell lines.
Matsuzaki E, Takahashi-Yanaga F, Miwa Y, Hirata M, Watanabe Y, Sato N, Morimoto S, Hirofuji T, Maeda K, Sasaguri T. Matsuzaki E, et al. J Bone Miner Res. 2006 Aug;21(8):1307-16. doi: 10.1359/jbmr.060512. J Bone Miner Res. 2006. PMID: 16869729 - COL11A1/(pro)collagen 11A1 expression is a remarkable biomarker of human invasive carcinoma-associated stromal cells and carcinoma progression.
Vázquez-Villa F, García-Ocaña M, Galván JA, García-Martínez J, García-Pravia C, Menéndez-Rodríguez P, González-del Rey C, Barneo-Serra L, de Los Toyos JR. Vázquez-Villa F, et al. Tumour Biol. 2015 Apr;36(4):2213-22. doi: 10.1007/s13277-015-3295-4. Epub 2015 Mar 12. Tumour Biol. 2015. PMID: 25761876 Review.
Cited by
- Collagen Type XI Alpha 1 (COL11A1): A Novel Biomarker and a Key Player in Cancer.
Nallanthighal S, Heiserman JP, Cheon DJ. Nallanthighal S, et al. Cancers (Basel). 2021 Feb 24;13(5):935. doi: 10.3390/cancers13050935. Cancers (Basel). 2021. PMID: 33668097 Free PMC article. Review. - Looping mediated interaction between the promoter and 3' UTR regulates type II collagen expression in chondrocytes.
Jash A, Yun K, Sahoo A, So JS, Im SH. Jash A, et al. PLoS One. 2012;7(7):e40828. doi: 10.1371/journal.pone.0040828. Epub 2012 Jul 16. PLoS One. 2012. PMID: 22815835 Free PMC article. - Nuclear factor Y (NF-Y) regulates the proximal promoter activity of the mouse collagen α1(XI) gene (Col11a1) in chondrocytes.
Hida M, Hamanaka R, Okamoto O, Yamashita K, Sasaki T, Yoshioka H, Matsuo N. Hida M, et al. In Vitro Cell Dev Biol Anim. 2014 Apr;50(4):358-66. doi: 10.1007/s11626-013-9692-3. Epub 2013 Oct 3. In Vitro Cell Dev Biol Anim. 2014. PMID: 24092017 - Gene markers of cellular aging in human multipotent stromal cells in culture.
Bellayr IH, Catalano JG, Lababidi S, Yang AX, Lo Surdo JL, Bauer SR, Puri RK. Bellayr IH, et al. Stem Cell Res Ther. 2014 Apr 28;5(2):59. doi: 10.1186/scrt448. Stem Cell Res Ther. 2014. PMID: 24780490 Free PMC article. - Integrated network analysis to explore the key genes regulated by parathyroid hormone receptor 1 in osteosarcoma.
Guan D, Tian H. Guan D, et al. World J Surg Oncol. 2017 Sep 21;15(1):177. doi: 10.1186/s12957-017-1242-0. World J Surg Oncol. 2017. PMID: 28934958 Free PMC article.
References
- Annunen S, Korkko J, Czarny M, Warman ML, Brunner HG, Kaariainen H, Mulliken JB, Tranebjaerg L, Brooks DG, Cox GF, Cruysberg JR, Curtis MA, Davenport SL, Friedrich CA, Kaitila I, Krawczynski MR, Latos-Bielenska A, Mukai S, Olsen BR, Shinno N, Somer M, Vikkula M, Zlotogora J, Prockop DJ, Ala-Kokko L. Splicing mutations of 54-bp exons in the COL11A1 gene cause Marshall syndrome, but other mutations cause overlapping Marshall/Stickler phenotypes. Am. J. Hum. Genet. 1999;65:974–983. - PMC - PubMed
- Baron R, Rawadi G, Roman-Roman S. Wnt signaling: a key regulator of bone mass. Curr. Top. Dev. Biol. 2006;76:103–127. - PubMed
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
- Bi Y, Stuelten CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG, Chen XD, Young MF. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J. Biol. Chem. 2005;280:30481–30489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR47985/AR/NIAMS NIH HHS/United States
- R01 AR047985-02/AR/NIAMS NIH HHS/United States
- R01 AR050074-02/AR/NIAMS NIH HHS/United States
- T32-AR050938/AR/NIAMS NIH HHS/United States
- AR050074/AR/NIAMS NIH HHS/United States
- R01 AR047985-03/AR/NIAMS NIH HHS/United States
- R01 AR050074-04/AR/NIAMS NIH HHS/United States
- P20 RR016454-096114/RR/NCRR NIH HHS/United States
- R01 AR047985-05/AR/NIAMS NIH HHS/United States
- R01 AR047985/AR/NIAMS NIH HHS/United States
- AR48672/AR/NIAMS NIH HHS/United States
- P20 RR016454-086155/RR/NCRR NIH HHS/United States
- K02 AR048672-05/AR/NIAMS NIH HHS/United States
- K02 AR048672-02/AR/NIAMS NIH HHS/United States
- R01 AR048147/AR/NIAMS NIH HHS/United States
- R01 AR047985-01/AR/NIAMS NIH HHS/United States
- AR048147/AR/NIAMS NIH HHS/United States
- K02 AR048672/AR/NIAMS NIH HHS/United States
- K02 AR048672-01/AR/NIAMS NIH HHS/United States
- R01 AR047985-04/AR/NIAMS NIH HHS/United States
- R01 AR050074-03/AR/NIAMS NIH HHS/United States
- T32 AR050938/AR/NIAMS NIH HHS/United States
- P20 RR016454/RR/NCRR NIH HHS/United States
- R01 AR050074-05/AR/NIAMS NIH HHS/United States
- R01 AR050074/AR/NIAMS NIH HHS/United States
- K02 AR048672-04/AR/NIAMS NIH HHS/United States
- K02 AR048672-03/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous