Microenvironmental regulation of cancer development - PubMed (original) (raw)
Review
Microenvironmental regulation of cancer development
Min Hu et al. Curr Opin Genet Dev. 2008 Feb.
Abstract
Numerous studies have demonstrated that the tumor microenvironment not only responds to and supports carcinogenesis, but also actively contributes to tumor initiation, progression, and metastasis. During tumor progression all cells composing the tumor undergo phenotypic and epigenetic changes. Paracrine signaling between epithelial and stromal cells is important for the regulation of the proliferation, invasive, angiogenic, and metastatic behavior of cancer cells. Better understanding the molecular mechanisms by which stromal cells exert these effects may open up new venues for cancer therapeutic and preventative interventions.
Figures
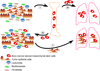
Figure 1. The dual role of bone marrow derived mesenchymal stem cells (BMD-MSCs) in cancer metastasis
1. Primary tumor cells send signals to the bone marrow mobilizing BMD-MSCs. 2. BMD-MSCs are recruited to the primary tumor (2a), and also mobilized to other organs such as lungs (2b), creating a pre-metastatic niche prior to the dissemination of the cancer cells. 3. The interaction between cancer cells and BMD-MSCs within the primary tumor enhances the motility, invasive and metastatic capacity of tumor cells via paracrine interactions (e.g., CCL5-CCR5). In addition, BMD-MSCs can differentiate into myofibroblasts and other stromal cell types that further support the growth and progression of the tumor. Furthermore, disseminated cancer cells preferentially grow at sites where BMD-MSCs are localized forming distant metastases.
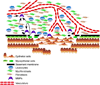
Figure 2. Events involved in the in situ to invasive breast carcinoma transition
Progression to invasion is marked by the disappearance of myoepithelial layer, disruption of the basement membrane, accompanied by infiltration of leukocytes, accumulation of myofibroblasts, and intrusion of capillary vessels. High levels of MMPs produced by DCIS associated myoepithelial cells, fibroblasts, myofibroblasts, and leukocytes all contribute to the degradation of the basement membrane and tumor progression.
Similar articles
- The epigenetic basis of the Warburg effect.
Wang X, Jin H. Wang X, et al. Epigenetics. 2010 Oct 1;5(7):566-8. doi: 10.4161/epi.5.7.12662. Epub 2010 Oct 1. Epigenetics. 2010. PMID: 20622527 Review. - Paracrine Fibroblast Growth Factor Initiates Oncogenic Synergy with Epithelial FGFR/Src Transformation in Prostate Tumor Progression.
Li Q, Ingram L, Kim S, Beharry Z, Cooper JA, Cai H. Li Q, et al. Neoplasia. 2018 Mar;20(3):233-243. doi: 10.1016/j.neo.2018.01.006. Epub 2018 Feb 11. Neoplasia. 2018. PMID: 29444487 Free PMC article. - Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer.
Joesting MS, Perrin S, Elenbaas B, Fawell SE, Rubin JS, Franco OE, Hayward SW, Cunha GR, Marker PC. Joesting MS, et al. Cancer Res. 2005 Nov 15;65(22):10423-30. doi: 10.1158/0008-5472.CAN-05-0824. Cancer Res. 2005. PMID: 16288033 - Co-evolution of tumor cells and their microenvironment.
Polyak K, Haviv I, Campbell IG. Polyak K, et al. Trends Genet. 2009 Jan;25(1):30-8. doi: 10.1016/j.tig.2008.10.012. Epub 2008 Dec 4. Trends Genet. 2009. PMID: 19054589 Review. - The roles of TGFβ in the tumour microenvironment.
Pickup M, Novitskiy S, Moses HL. Pickup M, et al. Nat Rev Cancer. 2013 Nov;13(11):788-99. doi: 10.1038/nrc3603. Epub 2013 Oct 17. Nat Rev Cancer. 2013. PMID: 24132110 Free PMC article. Review.
Cited by
- Carboxyamidotriazole-orotate inhibits the growth of imatinib-resistant chronic myeloid leukaemia cells and modulates exosomes-stimulated angiogenesis.
Corrado C, Flugy AM, Taverna S, Raimondo S, Guggino G, Karmali R, De Leo G, Alessandro R. Corrado C, et al. PLoS One. 2012;7(8):e42310. doi: 10.1371/journal.pone.0042310. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22879938 Free PMC article. Retracted. - Extracellular matrix composition defines an ultra-high-risk group of neuroblastoma within the high-risk patient cohort.
Tadeo I, Berbegall AP, Castel V, García-Miguel P, Callaghan R, Påhlman S, Navarro S, Noguera R. Tadeo I, et al. Br J Cancer. 2016 Aug 9;115(4):480-9. doi: 10.1038/bjc.2016.210. Epub 2016 Jul 14. Br J Cancer. 2016. PMID: 27415013 Free PMC article. - Molecular diversity in ductal carcinoma in situ (DCIS) and early invasive breast cancer.
Muggerud AA, Hallett M, Johnsen H, Kleivi K, Zhou W, Tahmasebpoor S, Amini RM, Botling J, Børresen-Dale AL, Sørlie T, Wärnberg F. Muggerud AA, et al. Mol Oncol. 2010 Aug;4(4):357-68. doi: 10.1016/j.molonc.2010.06.007. Epub 2010 Jun 26. Mol Oncol. 2010. PMID: 20663721 Free PMC article. - Targeting chronic lymphocytic leukemia with N-methylated thrombospondin-1-derived peptides overcomes drug resistance.
Pramil E, Herbi Bastian L, Denèfle T, Nemati F, Xiao M, Lardé E, Maloum K, Roos-Weil D, Chapiro E, Le Garff-Tavernier M, Davi F, Decaudin D, Sarfati M, Nguyen-Khac F, Merle-Béral H, Karoyan P, Susin SA. Pramil E, et al. Blood Adv. 2019 Oct 22;3(20):2920-2933. doi: 10.1182/bloodadvances.2019000350. Blood Adv. 2019. PMID: 31648314 Free PMC article. - Co-evolution of cancer microenvironment reveals distinctive patterns of gastric cancer invasion: laboratory evidence and clinical significance.
Peng CW, Liu XL, Liu X, Li Y. Peng CW, et al. J Transl Med. 2010 Oct 15;8:101. doi: 10.1186/1479-5876-8-101. J Transl Med. 2010. PMID: 20950454 Free PMC article.
References
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. - PubMed
- Radisky D, Hagios C, Bissell MJ. Tumors are unique organs defined by abnormal signaling and context. Semin Cancer Biol. 2001;11:87–95. - PubMed
- Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev. 2001;11:54–59. - PubMed
- Weinberg R, Mihich E. Eighteenth annual pezcoller symposium: tumor microenvironment and heterotypic interactions. Cancer Res. 2006;66:11550–11553. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA116235-01A1/CA/NCI NIH HHS/United States
- P50 CA089393-080014/CA/NCI NIH HHS/United States
- P50 CA089393-040004/CA/NCI NIH HHS/United States
- P50 CA089393-020004/CA/NCI NIH HHS/United States
- P50 CA089393-030004/CA/NCI NIH HHS/United States
- P50 CA089393-060014/CA/NCI NIH HHS/United States
- R01 CA116235-02/CA/NCI NIH HHS/United States
- CA94074/CA/NCI NIH HHS/United States
- CA89393/CA/NCI NIH HHS/United States
- P50 CA089393-010004/CA/NCI NIH HHS/United States
- CA116235/CA/NCI NIH HHS/United States
- R01 CA116235-03/CA/NCI NIH HHS/United States
- P50 CA089393/CA/NCI NIH HHS/United States
- R01 CA116235/CA/NCI NIH HHS/United States
- P50 CA089393-070014/CA/NCI NIH HHS/United States
- R01 CA094074/CA/NCI NIH HHS/United States
- P50 CA089393-050004/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources