The yeast p24 complex is required for the formation of COPI retrograde transport vesicles from the Golgi apparatus - PubMed (original) (raw)
The yeast p24 complex is required for the formation of COPI retrograde transport vesicles from the Golgi apparatus
Auxiliadora Aguilera-Romero et al. J Cell Biol. 2008.
Abstract
The p24 family members are transmembrane proteins assembled into heteromeric complexes that continuously cycle between the ER and the Golgi apparatus. These cargo proteins were assumed to play a structural role in COPI budding because of their major presence in mammalian COPI vesicles. However, this putative function has not been proved conclusively so far. Furthermore, deletion of all eight yeast p24 family members does not produce severe transport phenotypes, suggesting that the p24 complex is not essential for COPI function. In this paper we provide direct evidence that the yeast p24 complex plays an active role in retrograde transport from Golgi to ER by facilitating the formation of COPI-coated vesicles. Therefore, our results demonstrate that p24 proteins are important for vesicle formation instead of simply being a passive traveler, supporting the model in which cargo together with a small GTPase of the ARF superfamily and coat subunits act as primer for vesicle formation.
Figures
Figure 1.
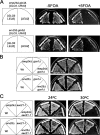
The emp24Δ and erv25Δ mutations interact genetically with mutant alleles of genes encoding for COPI vesicle coat components. (A) The emp24Δ glo3Δ strain with the URA3 based plasmid bearing GLO3 pMMY63 was transformed with _LEU2_-based empty vector pRS315 or GLO3 encoding plasmid pMMY67. Double transformants were replica plated at 30°C on SD-leu-ura or on 5-FOA medium to induce loss of pMMY63. (B) The emp24Δ or erv25Δ strains were crossed with a gcs1Δ strain and the resulting haploid spores were tested for growth at 30°C on YPUAD. (C) emp24Δ or erv25Δ strains were crossed with a sec21-1 strain and the resulting haploid spores were tested for growth at 24 and 30°C and grown on YPUAD.
Figure 2.
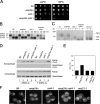
The emp24Δ ret4-1 double mutant cells exhibit COPI mutant–like phenotypes. (A) emp24Δ strain was crossed with a ret4-1 strain and the resulting haploid spores were tested for growth at 24 and 35°C on YPUAD. (B) Proliferating cells were radiolabeled for 5 min, chased for 30 min at 24°C, and lysed. CPY was immunoprecipitated, resolved by SDS-PAGE, and analyzed by PhosphoImager. ER (p1), Golgi (p2), and vacuole (m) CPY forms are indicated. (C) Low glucose–induced cells were pulse-labeled for 5 min and chased for 30 min at 24°C. Cells were converted to spheroplasts, and separated into intracellular (I) and extracellular (E) fractions from which invertase was immunoprecipitated, resolved by SDS-PAGE, and analyzed by PhosphoImager. Migration positions of core glycosylated, hypo-, and hyperglycosylated invertase are indicated. (D) Cells without (1, 2, 3, and 4) or with (5, 6, 7, and 8) ERD2 2μ plasmid (pJS209) were transferred to fresh media for 1 h at 24°C. Proteins contained in the cell culture supernatant were concentrated by TCA precipitation, resolved by SDS–PAGE, and Kar2p and Hexokinase were detected by immunoblotting. (E) β-Galactosidase assays were performed on strains harboring the reporter construct (pJC31). (F) Fluorescence images of cells expressing the ER-localized protein GFP-HDEL. Cells were grown at 24°C, except sec21-1 cells, which were grown at 24°C and then shifted 40 min at 37°C. Bar, 5 μm.
Figure 3.
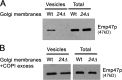
Golgi membranes derived from emp24Δ mutant cells are defective for the generation of COPI vesicles in vitro. Vesicles were generated from wild-type and emp24Δ (RH4443) Golgi membranes, which were incubated with GTP in the absence (A) or the presence of an excess of COPI components (B). The vesicles were purified over a velocity gradient, and subsequently floated on a Nycodenz gradient. Vesicle-containing fractions were collected and pooled, TCA precipitated, resolved on SDS-PAGE, and analyzed by immunoblot using antibody against Emp47p.
Figure 4.
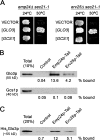
Specific genetic and physical interactions between p24 members and COPI coat components. (A) The emp24Δ sec21-1 and erv25_Δ sec21-1_ cells were transformed with empty plasmid (YEp352) or a high-copy plasmid carrying either the GLO3 (pPPL43) or the GCS1 gene (pPP421). Transformed cells were tested for growth at 24 and 30°C. (B and C) Synthetic peptides corresponding to cytoplasmic domains of Emp24p (RRFFEVTSLV) and Erv25p (KNYFKTKHII) were coupled to thiopropyl-Sepharose beads and incubated with cytosol (B) or recombinant Glo3p (C). Bound material was resolved by SDS-PAGE and analyzed by immunoblot using antibodies against Glo3p and Gcs1p.
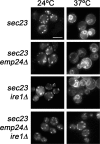
Figure 5.
UPR compensates the loss of retrograde transport function in the absence of p24 proteins. Cells expressing Rer1p-GFP were observed by fluorescence microscopy at 24°C, or after the shift to 37°C for 20 min. Bar, 5 μm.
Similar articles
- The p24 Complex Contributes to Specify Arf1 for COPI Coat Selection.
Sabido-Bozo S, Perez-Linero AM, Manzano-Lopez J, Rodriguez-Gallardo S, Aguilera-Romero A, Cortes-Gomez A, Lopez S, Wellinger RE, Muñiz M. Sabido-Bozo S, et al. Int J Mol Sci. 2021 Jan 3;22(1):423. doi: 10.3390/ijms22010423. Int J Mol Sci. 2021. PMID: 33401608 Free PMC article. - Trs65p, a subunit of the Ypt1p GEF TRAPPII, interacts with the Arf1p exchange factor Gea2p to facilitate COPI-mediated vesicle traffic.
Chen S, Cai H, Park SK, Menon S, Jackson CL, Ferro-Novick S. Chen S, et al. Mol Biol Cell. 2011 Oct;22(19):3634-44. doi: 10.1091/mbc.E11-03-0197. Epub 2011 Aug 3. Mol Biol Cell. 2011. PMID: 21813735 Free PMC article. - Auxilin facilitates membrane traffic in the early secretory pathway.
Ding J, Segarra VA, Chen S, Cai H, Lemmon SK, Ferro-Novick S. Ding J, et al. Mol Biol Cell. 2016 Jan 1;27(1):127-36. doi: 10.1091/mbc.E15-09-0631. Epub 2015 Nov 4. Mol Biol Cell. 2016. PMID: 26538028 Free PMC article. - [The p24 family proteins--regulators of vesicular trafficking].
Kamińska J, Hoffman-Sommer M, Płachta M. Kamińska J, et al. Postepy Biochem. 2010;56(1):75-82. Postepy Biochem. 2010. PMID: 20499684 Review. Polish. - Formation of COPI-coated vesicles at a glance.
Arakel EC, Schwappach B. Arakel EC, et al. J Cell Sci. 2018 Mar 13;131(5):jcs209890. doi: 10.1242/jcs.209890. J Cell Sci. 2018. PMID: 29535154 Review.
Cited by
- Arf GAPs: gatekeepers of vesicle generation.
Spang A, Shiba Y, Randazzo PA. Spang A, et al. FEBS Lett. 2010 Jun 18;584(12):2646-51. doi: 10.1016/j.febslet.2010.04.005. Epub 2010 Apr 13. FEBS Lett. 2010. PMID: 20394747 Free PMC article. Review. - Putative p24 complexes in Arabidopsis contain members of the delta and beta subfamilies and cycle in the early secretory pathway.
Montesinos JC, Langhans M, Sturm S, Hillmer S, Aniento F, Robinson DG, Marcote MJ. Montesinos JC, et al. J Exp Bot. 2013 Aug;64(11):3147-67. doi: 10.1093/jxb/ert157. J Exp Bot. 2013. PMID: 23918961 Free PMC article. - A Kinetic View of Membrane Traffic Pathways Can Transcend the Classical View of Golgi Compartments.
Pantazopoulou A, Glick BS. Pantazopoulou A, et al. Front Cell Dev Biol. 2019 Aug 6;7:153. doi: 10.3389/fcell.2019.00153. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31448274 Free PMC article. Review. - Sorting of GPI-anchored proteins into ER exit sites by p24 proteins is dependent on remodeled GPI.
Fujita M, Watanabe R, Jaensch N, Romanova-Michaelides M, Satoh T, Kato M, Riezman H, Yamaguchi Y, Maeda Y, Kinoshita T. Fujita M, et al. J Cell Biol. 2011 Jul 11;194(1):61-75. doi: 10.1083/jcb.201012074. Epub 2011 Jul 4. J Cell Biol. 2011. PMID: 21727194 Free PMC article. - Dual Independent Roles of the p24 Complex in Selectivity of Secretory Cargo Export from the Endoplasmic Reticulum.
Lopez S, Perez-Linero AM, Manzano-Lopez J, Sabido-Bozo S, Cortes-Gomez A, Rodriguez-Gallardo S, Aguilera-Romero A, Goder V, Muñiz M. Lopez S, et al. Cells. 2020 May 22;9(5):1295. doi: 10.3390/cells9051295. Cells. 2020. PMID: 32456004 Free PMC article.
References
- Belden, W.J., and C. Barlowe. 2001. b. Distinct roles for the cytoplasmic tail sequences of Emp24p and Erv25p in transport between the endoplasmic reticulum and Golgi complex. J. Biol. Chem. 276:43040–43048. - PubMed
- Bremser, M., W. Nickel, M. Schweikert, M. Ravazzola, M. Amherdt, C.A. Hughes, T.H. Sollner, J.E. Rothman, and F.T. Wieland. 1999. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 96:495–506. - PubMed
- Cox, J.S., and P. Walter. 1996. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 87:391–404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases