Role of the TMPRSS2-ERG gene fusion in prostate cancer - PubMed (original) (raw)
Bharathi Laxman, Sooryanarayana Varambally, Xuhong Cao, Jindan Yu, Beth E Helgeson, Qi Cao, John R Prensner, Mark A Rubin, Rajal B Shah, Rohit Mehra, Arul M Chinnaiyan
Affiliations
- PMID: 18283340
- PMCID: PMC2244693
- DOI: 10.1593/neo.07822
Role of the TMPRSS2-ERG gene fusion in prostate cancer
Scott A Tomlins et al. Neoplasia. 2008 Feb.
Abstract
TMPRSS2-ERG gene fusions are the predominant molecular subtype of prostate cancer. Here, we explored the role of TMPRSS2-ERG gene fusion product using in vitro and in vivo model systems. Transgenic mice expressing the ERG gene fusion product under androgen-regulation develop mouse prostatic intraepithelial neoplasia (PIN), a precursor lesion of prostate cancer. Introduction of the ERG gene fusion product into primary or immortalized benign prostate epithelial cells induced an invasion-associated transcriptional program but did not increase cellular proliferation or anchorage-independent growth. These results suggest that TMPRSS2-ERG may not be sufficient for transformation in the absence of secondary molecular lesions. Transcriptional profiling of ERG knockdown in the TMPPRSS2-ERG-positive prostate cancer cell line VCaP revealed decreased expression of genes over-expressed in prostate cancer versus PIN and genes overexpressed in ETS-positive versus -negative prostate cancers in addition to inhibiting invasion. ERG knockdown in VCaP cells also induced a transcriptional program consistent with prostate differentiation. Importantly, VCaP cells and benign prostate cells overexpressing ERG directly engage components of the plasminogen activation pathway to mediate cellular invasion, potentially representing a downstream ETS target susceptible to therapeutic intervention. Our results support previous work suggesting that TMPRSS2-ERG fusions mediate invasion, consistent with the defining histologic distinction between PIN and prostate cancer.
Figures
Figure 1
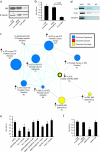
Transgenic mice recapitulating TMPRSS2-ERG in the prostate develop mPIN. (a) To recapitulate TMPRSS2-ERG in vivo, we generated transgenic mice over-expressing the ERG gene fusion product (exons 2 through the reported stop codon; 1533 of NM_182918.2, C-terminal 3X-FLAG epitope tag) with a bovine growth hormone polyA signal (PA-BGH) under the control of the enhanced probasin promoter (ARR2Pb). Mice were sacrificed at 12 to 14 weeks or >20 weeks, and mouse prostatic intraepithelial neoplasia (mPIN) was observed in 4 of 11 ARR2Pb-ERG mice as described in Table W1. Benign epithelia and areas of mPIN are indicated by yellow and black arrows, respectively. (b–d) Hematoxylin and eosin staining of ARR2Pb-ERG prostates for morphologic assessment. Consistent with the focality of mPIN, (b) benign glands and (c and d) mPIN were observed in the ventral prostate (VP) of ARR2Pb-ERG mice. Original magnification: (b) ×400, (c) ×200, and (d) inset showing area of mPIN with macronucleoli, ×400.
Figure 2
Over-expression of ERG in RWPE cells increases invasion through the plasminogen activator pathway. (a) To recapitulate TMPRSS2-ERG in vitro, we generated adenoviruses and lentiviruses expressing the ERG gene fusion product (exons 2 through the reported stop codon). (b and c) Infected (b) RWPE and (c) PrEC cells as indicated were assayed for invasion through a modified basement membrane. Photomicrographs of invaded cells are shown below. (d) RWPE-ERG and RWPE-GUS (control vector) cells were profiled on Agilent Whole Genome microarrays and expression signatures were loaded into the Oncomine Concept Map. Molecular concept map analysis of the over-expressed in RWPE-ERG compared to RWPE-GUS signature (ringed yellow node). Each node represents a molecular concept, or set of biologically related genes. The node size is proportional to the number of genes in the concept. The concept color indicates the concept type according to the legend. Each edge represents a significant enrichment (P < .005). (e) qPCR confirmation of increased expression of genes involved in invasion. The amount of the indicated gene (normalized to the average of GAPDH and HMBS) in RWPE-GUS (white) and RWPE-ERG (black) is shown. Inset shows immunoblot confirmation of increased expression of PLAU and MMP3 in RWPE-ERG cells. (f) Chromatin immunoprecipitation shows enrichment of ERG binding to the proximal promoters of PLAU and MMP3 compared to IgG control. The promoter of KIAA0089 was used as a negative control. (g) RWPE-ERG cells were treated with PLAU inhibitors amiloride or ectopic PAI-1, MMP inhibitors (including the pan-MMP inhibitor GM-6001), or the EWS:FLI inhibitor ARA-C (EWS:FLI inhibitor) as indicated and assayed for invasion as in c. (h) RWPE-ERG cells were treated with transfection reagent alone (untreated), or transfected with nontargeting, PLAU or PLAT siRNA as indicated and assayed for invasion through a modified basement membrane. For all invasion assays, mean (n = 3) ± SEM are shown; *P < .05.31
Figure 3
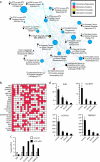
Knockdown of ERG in VCaP cells attenuates a transcriptional program over-expressed in TMPRSS2-ETS_-positive prostate cancers. (a) SiRNA knockdown of ERG in the TMPRSS2-ERG_-positive prostate cancer cell line VCaP. VCaP cells were treated with transfection reagent alone (untreated), or transfected with nontargeting or ERG siRNA (VCaP-si_ERG) as indicated. ERG knockdown was confirmed by immunoblot analysis. (b) VCaP cells as indicated were assayed for invasion through a modified basement membrane. (c) VCaP-si_ERG and VCaP cells treated with nontargeting siRNA were profiled and a molecular concept map of the under-expressed in VCaP-si_ERG_ signature (ringed yellow node) was generated. Each edge represents a significant enrichment (P < .001). Blue edges indicate enrichments with in vivo ETS_-positive versus negative prostate cancer signatures. (d) Chromatin immunoprecipitation identifies PLAT and PLAU as direct targets of ERG in VCaP cells, by enrichment of ERG binding to the proximal promoters of PLAT and PLAU compared to IgG control. The promoter of KIAA0089 was used as a negative control. (e) VCaP cells were treated with the indicated inhibitors (as in Figure 2_g) and assessed for invasion. (f) VCaP cells were treated with transfection reagent alone (untreated), or transfected with nontargeting, PLAU or PLAT siRNA as indicated and assayed for invasion. For all invasion assays, mean (n = 3) ± SEM are shown; *P < .05.
Figure 4
ERG knockdown in VCaP cells derepresses a transcriptional program associated with normal prostatic epithelial cell differentiation. (a) VCaP-si_ERG_ and VCaP cells treated with nontargeting siRNA were profiled and a molecular concept map of the over-expressed in VCaP-siERG signature (ringed yellow node) was generated. Each edge represents a significant enrichment (P < .001). Blue edges indicate enrichments with in vivo ETS-positive versus -negative prostate cancer signatures. (b) Overlay map identifying genes present (red cells), including KLK3 (PSA), across multiple concepts in the over-expressed in VCaP-si_ERG_ enrichment network (indicated by number). (c) qPCR confirmation of increased expression in VCaP-si_ERG_ cells (black) compared to VCaP-NT cells (white) of transcripts strongly expressed in prostatic epithelial cells. (d) Analysis of prostate cell type specificity using a microarray data set profiling magnetically sorted prostate cell populations. Mean RMA normalized fluorescent intensities (n = 5 ± SEM) are shown. *P < .05, for all pairwise t tests involving luminal cells.
Similar articles
- TMPRSS2- driven ERG expression in vivo increases self-renewal and maintains expression in a castration resistant subpopulation.
Casey OM, Fang L, Hynes PG, Abou-Kheir WG, Martin PL, Tillman HS, Petrovics G, Awwad HO, Ward Y, Lake R, Zhang L, Kelly K. Casey OM, et al. PLoS One. 2012;7(7):e41668. doi: 10.1371/journal.pone.0041668. Epub 2012 Jul 30. PLoS One. 2012. PMID: 22860005 Free PMC article. - TMPRSS2:ERG fusion by translocation or interstitial deletion is highly relevant in androgen-dependent prostate cancer, but is bypassed in late-stage androgen receptor-negative prostate cancer.
Hermans KG, van Marion R, van Dekken H, Jenster G, van Weerden WM, Trapman J. Hermans KG, et al. Cancer Res. 2006 Nov 15;66(22):10658-63. doi: 10.1158/0008-5472.CAN-06-1871. Cancer Res. 2006. PMID: 17108102 - Mapping of TMPRSS2-ERG fusions in the context of multi-focal prostate cancer.
Furusato B, Gao CL, Ravindranath L, Chen Y, Cullen J, McLeod DG, Dobi A, Srivastava S, Petrovics G, Sesterhenn IA. Furusato B, et al. Mod Pathol. 2008 Feb;21(2):67-75. doi: 10.1038/modpathol.3800981. Epub 2007 Dec 7. Mod Pathol. 2008. PMID: 18065961 - Clinical applications of novel ERG immunohistochemistry in prostate cancer diagnosis and management.
Shah RB. Shah RB. Adv Anat Pathol. 2013 Mar;20(2):117-24. doi: 10.1097/PAP.0b013e3182862ac5. Adv Anat Pathol. 2013. PMID: 23399797 Review. - [The progress of TMPRSS2-ETS gene fusions and their mechanism in prostate cancer].
Guo XQ, Gui YT, Cai ZM. Guo XQ, et al. Yi Chuan. 2011 Feb;33(2):117-22. doi: 10.3724/sp.j.1005.2011.00117. Yi Chuan. 2011. PMID: 21377967 Review. Chinese.
Cited by
- The L-type calcium channel CaV1.3: A potential target for cancer therapy.
Liu X, Shen B, Zhou J, Hao J, Wang J. Liu X, et al. J Cell Mol Med. 2024 Oct;28(19):e70123. doi: 10.1111/jcmm.70123. J Cell Mol Med. 2024. PMID: 39365143 Free PMC article. Review. - Prostate Cancer Progression Modeling Provides Insight into Dynamic Molecular Changes Associated with Progressive Disease States.
Chen R, Tang L, Melendy T, Yang L, Goodison S, Sun Y. Chen R, et al. Cancer Res Commun. 2024 Oct 1;4(10):2783-2798. doi: 10.1158/2767-9764.CRC-24-0210. Cancer Res Commun. 2024. PMID: 39347576 Free PMC article. - Potential Effects of Hyperglycemia on SARS-CoV-2 Entry Mechanisms in Pancreatic Beta Cells.
Michaels TM, Essop MF, Joseph DE. Michaels TM, et al. Viruses. 2024 Aug 2;16(8):1243. doi: 10.3390/v16081243. Viruses. 2024. PMID: 39205219 Free PMC article. Review. - RAD21 promotes oncogenesis and lethal progression of prostate cancer.
Su XA, Stopsack KH, Schmidt DR, Ma D, Li Z, Scheet PA, Penney KL, Lotan TL, Abida W, DeArment EG, Lu K, Janas T, Hu S, Vander Heiden MG, Loda M, Boselli M, Amon A, Mucci LA. Su XA, et al. Proc Natl Acad Sci U S A. 2024 Sep 3;121(36):e2405543121. doi: 10.1073/pnas.2405543121. Epub 2024 Aug 27. Proc Natl Acad Sci U S A. 2024. PMID: 39190349 Free PMC article. - TMPRSS2 is a tumor suppressor and its downregulation promotes antitumor immunity and immunotherapy response in lung adenocarcinoma.
Liu Z, Lu Q, Zhang Z, Feng Q, Wang X. Liu Z, et al. Respir Res. 2024 Jun 11;25(1):238. doi: 10.1186/s12931-024-02870-7. Respir Res. 2024. PMID: 38862975 Free PMC article.
References
- Tomlins SA, Mehra R, Rhodes DR, Smith LR, Roulston D, Helgeson BE, Cao X, Wei JT, Rubin MA, Shah RB, et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 2006;66:3396–3400. - PubMed
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. - PubMed
- Helgeson BE, Tomlins SA, Shah N, Laxman B, Cao Q, Prensner JR, Cao X, Singla N, Montie JE, Varambally S, et al. Characterization of TMPRSS2:ETV5 and SLC45A3:ETV5 gene fusions in prostate cancer. Cancer Res. 2008;68:73–80. - PubMed
- Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, Hoshida Y, Mosquera JM, Pawitan Y, Lee C, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene - PubMed
- Lapointe J, Kim YH, Miller MA, Li C, Kaygusuz G, van de Rijn M, Huntsman DG, Brooks JD, Pollack JR. A variant TMPRSS2 isoform and ERG fusion product in prostate cancer with implications for molecular diagnosis. Mod Pathol. 2007;20:467–473. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases