Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1 - PubMed (original) (raw)
Comparative Study
Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1
Surya M Nauli et al. Circulation. 2008.
Abstract
Background: When challenged with extracellular fluid shear stress, vascular endothelial cells are known to release nitric oxide, an important vasodilator. Here, we show that the ability of cultured endothelial cells to sense a low range of fluid shear depends on apical membrane organelles, called cilia, and that cilia are compartments required for proper localization and function of the mechanosensitive polycystin-1 molecule.
Methods and results: Cells with the Pkd1(null/null) or Tg737(orpk/orpk) mutation encoded for polycystin-1 or polaris, respectively, are unable to transmit extracellular shear stress into intracellular calcium signaling and biochemical nitric oxide synthesis. Cytosolic calcium and nitric oxide recordings further show that fluid shear sensing is a cilia-specific mechanism because other mechanical or pharmacological stimulation does not abolish calcium and nitric oxide signaling in polycystin-1 and polaris mutant endothelial cells. Polycystin-1 localized in the basal body of Tg737(orpk/orpk) endothelial cells is insufficient for a fluid shear stress response. Furthermore, the optimal shear stress to which the cells respond best does not alter the apical cilia structure but modifies the responsiveness of cells to higher shear stresses through proteolytic modification of polycystin-1.
Conclusions: We demonstrate for the first time that polycystin-1 (required for cilia function) and polaris (required for cilia structure) are crucial mechanosensitive molecules in endothelial cells. We propose that a distinctive communication with the extracellular microenvironment depends on the proper localization and function of polycystin-1 in cilia.
Figures
Figure 1
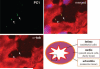
Polycystin-1 is localized in aortic endothelial cilia of embryonic mouse aorta. Isolated aorta from embryonic mouse was gently perfused with PBS to remove the remaining blood on the aorta wall and to help prevent the segment from collapsing. The aorta segment was cut to a thickness of 5 _μ_m. The segment was stained for polycystin-1 (PC1; green) and the ciliary marker acetylated _α_-tubulin (_α_-tub; red). A merged image with nuclear marker (blue) also is shown. Arrows indicate specific staining for polycystin-1 and cilia. Images were taken at a magnification of ×90. The figure at the bottom right represents the region of an aorta segment where the micrograph of the section was taken, designated by a black box.
Figure 2
Endothelial cells express ICAM-2 and eNOS and are regulated by temperature and IFN-γ. A, Fluorescence cell-sorting study shows that cultured endothelial cells from mouse embryonic aortas are positive for the endothelial marker ICAM-2 (open curves). Cells treated with secondary mouse antibody were used as a negative control (filled curves). n = 3. B, Endothelial cells under permissive conditions at 33°C in the presence of IFN-γ express SV40 large T antigen. When these cells are grown under nonpermissive conditions at 37°C in the absence of IFN-γ for 3 to 4 days, their expression of SV40 large T antigen can be suppressed. Human kidney (HK) cells were the negative control. Actin was used as the loading control. n = 1. C, Western blot analysis confirms the expression of eNOS in differentiated cells. Cells undergoing proliferation do not show eNOS expression. Mouse embryonic brain tissue was used as a positive control. n = 3.
Figure 3
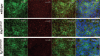
Endothelial cells express CD31 and have primary cilia. Cultured wild-type (top), Pkd1null/null (middle), and Tg737orpk/orpk (bottom) cells were stained for the endothelial marker CD31 (green), the ciliary marker acetylated _α_-tubulin (_α_-tub; red), and the nuclear marker DAPI (blue). Merged images with and without nuclear marker show that all cells were positive for CD31 and that each endothelial cell, except Tg737orpk/orpk cells, had 1 primary cilium. Bar=25 _μ_m. n = 2.
Figure 4
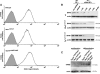
Endothelial cilia respond to a specific range of fluid shear stresses. A, Immunostaining studies show colocalization of polycystin-1 (PC1; green) and polaris (red) counterstained with nuclear marker DAPI (blue). Note that polycystin-1 appears to be concentrated in the basal body of the Tg737 mutant cells. Bar=5 _μ_m. n = 4. B, All cells were first exposed to a fluid shear stress of 0, 1.1, or 7.2 dynes/cm2 for 30 minutes, followed by the corresponding step increase in shear stress as depicted in the graphs. Cytosolic calcium ([Ca2+]cyt) was measured as a readout in response to fluid shear stress. Although wild-type cells respond to a range of shear stress, neither mutant cell shows any apparent response to different magnitudes of shear stress. n = 3 to 6 for each time point in a given shear stress condition. C, Cumulative measurements of cytosolic NO biosynthesis ([NO]cyt) at the predicted optimal ranges of shear stress. n = 3 to 6 for each time point in a given shear stress condition.
Figure 5
Optimal fluid shear preserves the presence of cilia and induces apparent proteolytic cleavage of polycystin-1. A, Endothelial cells equilibrated at an optimal shear stress of 7.2 dynes/cm2 for various time points maintain their apical primary cilia. The presence of cilia in every cell is confirmed with acetylated _α_-tubulin (acet-_α_-tubulin). Bar=10 _μ_m. n = 2 for each time point. B, Cells were equilibrated at 0, 1.1, or 7.2 dynes/cm2 for 10, 20, or 30 minutes. Only a single full-length polycystin-1 band is observed in static control cells (arrow), whereas fluid shear stress induces a second lower–molecular-weight polycystin-1 band (arrowhead). Note that the band intensity of full-length molecular-weight polycystin-1 in cells equilibrated at 7.2 dynes/cm2 is much weaker than that of static control or 1.1 dynes/cm2. n = 2.
Figure 6
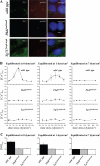
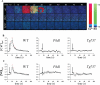
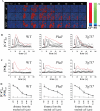
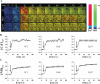
Fluid shear–induced calcium and transient NO require polaris and polycystin-1. A, Response of individual wild-type (wt), Pkd1null/null (Pkd1), and Tg737orpk/orpk (Tg737) cells from static condition to fluid shear stress of 7.2 dynes/cm2 is pseudocolored. The calcium (red) and NO (green) are superimposed. The color bar indicates the calcium (Ca2+) or NO level relative to the corresponding baseline level. Black and blue represent a low (lo) level; red and green denote a higher (hi) level of calcium and NO, respectively. B, The average cytosolic calcium ([Ca2+]cyt) response to shear stress. C, The average cytosolic NO ([NO]cyt) response. Fluid shear stress was applied after the baseline values of calcium and NO were obtained for at least 14 seconds as indicated by arrows. All the units of time are seconds. n = 5. *Statistically significant responses from the baseline values.
Figure 7
Mechanical touch–induced calcium propagation and NO production are independent of cilia. A, Response of individual wild-type (wt), Pkd1null/null (Pkd1), and Tg737orpk/orpk (Tg737) cells to a single apical cell pressing is pseudocolored. The calcium (red) and NO (green) are superimposed. The color bar indicates the Ca2+ or NO level relative to the corresponding baseline level, with black and blue representing a low (lo) level and red or green denoting a higher (hi) level. The apical membrane of a single cell (far left) was pressed after baseline values of calcium and NO were obtained for at least 14 seconds as indicated by arrows. B, The individual cytosolic calcium ([Ca2+]cyt) response to a mechanical stimulus of touching a single cell. C, The individual cytosolic NO ([NO]cyt) response. The red line in each graph represents the corresponding calcium and NO values of the touched cell. D, Cells were grouped on the basis of their distance away from the touched cells. The averages of their corresponding cytosolic calcium (•) and NO (○) responses are shown. The units of time and distance are seconds and microns, respectively. n = 3.
Figure 8
Agonist-provoked calcium and NO signaling is independent of cilia. A, Response of individual wild-type (wt), Pkd1null/null (Pkd1), and Tg737orpk/orpk (Tg737) cells to 1 _μ_mol/L acetylcholine is pseudocolored. The calcium (red) and NO (green) are superimposed. The color bar indicates the Ca2+ or NO level relative to the corresponding baseline level, with black and blue representing a low (lo) level and red or green denoting a higher (hi) level. B, The average cytosolic calcium ([Ca2+]cyt) response to acetylcholine. C, Plots of the average cytosolic NO ([NO]cyt) response. Acetylcholine was applied to the cells for at least 14 seconds after the baseline values of calcium and NO were obtained as indicated by arrows. All time units are seconds. n = 3.
Comment in
- Deciphering the endothelial shear stress sensor.
Poelmann RE, Van der Heiden K, Gittenberger-de Groot A, Hierck BP. Poelmann RE, et al. Circulation. 2008 Mar 4;117(9):1124-6. doi: 10.1161/CIRCULATIONAHA.107.753889. Circulation. 2008. PMID: 18316496 No abstract available.
Similar articles
- Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades.
AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM. AbouAlaiwi WA, et al. Circ Res. 2009 Apr 10;104(7):860-9. doi: 10.1161/CIRCRESAHA.108.192765. Epub 2009 Mar 5. Circ Res. 2009. PMID: 19265036 Free PMC article. - Non-motile primary cilia as fluid shear stress mechanosensors.
Nauli SM, Jin X, AbouAlaiwi WA, El-Jouni W, Su X, Zhou J. Nauli SM, et al. Methods Enzymol. 2013;525:1-20. doi: 10.1016/B978-0-12-397944-5.00001-8. Methods Enzymol. 2013. PMID: 23522462 Free PMC article. - The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia.
Yoder BK, Hou X, Guay-Woodford LM. Yoder BK, et al. J Am Soc Nephrol. 2002 Oct;13(10):2508-16. doi: 10.1097/01.asn.0000029587.47950.25. J Am Soc Nephrol. 2002. PMID: 12239239 - Polycystins and primary cilia: primers for cell cycle progression.
Zhou J. Zhou J. Annu Rev Physiol. 2009;71:83-113. doi: 10.1146/annurev.physiol.70.113006.100621. Annu Rev Physiol. 2009. PMID: 19572811 Review. - Structure and function of polycystin channels in primary cilia.
Ta CM, Vien TN, Ng LCT, DeCaen PG. Ta CM, et al. Cell Signal. 2020 Aug;72:109626. doi: 10.1016/j.cellsig.2020.109626. Epub 2020 Apr 3. Cell Signal. 2020. PMID: 32251715 Free PMC article. Review.
Cited by
- Established, New and Emerging Concepts in Brain Vascular Development.
Gupta A, Rarick KR, Ramchandran R. Gupta A, et al. Front Physiol. 2021 Feb 12;12:636736. doi: 10.3389/fphys.2021.636736. eCollection 2021. Front Physiol. 2021. PMID: 33643074 Free PMC article. Review. - Clinical findings, underlying pathogenetic processes and treatment of vascular dysfunction in autosomal dominant polycystic kidney disease.
Zhu J, Liu F, Mao J. Zhu J, et al. Ren Fail. 2023;45(2):2282027. doi: 10.1080/0886022X.2023.2282027. Epub 2023 Nov 16. Ren Fail. 2023. PMID: 37970664 Free PMC article. Review. - Update on vascular endothelial Ca(2+) signalling: A tale of ion channels, pumps and transporters.
Moccia F, Berra-Romani R, Tanzi F. Moccia F, et al. World J Biol Chem. 2012 Jul 26;3(7):127-58. doi: 10.4331/wjbc.v3.i7.127. World J Biol Chem. 2012. PMID: 22905291 Free PMC article. - Canonical TRP channels and mechanotransduction: from physiology to disease states.
Patel A, Sharif-Naeini R, Folgering JR, Bichet D, Duprat F, Honoré E. Patel A, et al. Pflugers Arch. 2010 Aug;460(3):571-81. doi: 10.1007/s00424-010-0847-8. Epub 2010 May 21. Pflugers Arch. 2010. PMID: 20490539 Review. - Lymphatic endothelial cell calcium pulses are sensitive to spatial gradients in wall shear stress.
Surya VN, Michalaki E, Fuller GG, Dunn AR. Surya VN, et al. Mol Biol Cell. 2019 Mar 21;30(7):923-931. doi: 10.1091/mbc.E18-10-0618. Epub 2019 Feb 27. Mol Biol Cell. 2019. PMID: 30811261 Free PMC article.
References
- Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol. 2002;282:F541–F552. - PubMed
- Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science. 1994;264:1329–1333. - PubMed
- Polycystic kidney disease: the complete structure of the PKD1 gene and its protein: the International Polycystic Kidney Disease Consortium. Cell. 1995;81:289–298. - PubMed
- Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays. 2004;26:844–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL064867/HL/NHLBI NIH HHS/United States
- P30 DK074038/DK/NIDDK NIH HHS/United States
- R01 DK051050/DK/NIDDK NIH HHS/United States
- R01 DK080640/DK/NIDDK NIH HHS/United States
- HL084451/HL/NHLBI NIH HHS/United States
- R01 DK040703/DK/NIDDK NIH HHS/United States
- R21 HL084451/HL/NHLBI NIH HHS/United States
- P01 CA045548/CA/NCI NIH HHS/United States
- R37 DK051050/DK/NIDDK NIH HHS/United States
- CA45548/CA/NCI NIH HHS/United States
- DK40703/DK/NIDDK NIH HHS/United States
- DK51050/DK/NIDDK NIH HHS/United States
- HL64867/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases