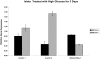The role of IRE1alpha in the degradation of insulin mRNA in pancreatic beta-cells - PubMed (original) (raw)
The role of IRE1alpha in the degradation of insulin mRNA in pancreatic beta-cells
Kathryn L Lipson et al. PLoS One. 2008.
Abstract
Background: The endoplasmic reticulum (ER) is a cellular compartment for the biosynthesis and folding of newly synthesized secretory proteins such as insulin. Perturbations to ER homeostasis cause ER stress and subsequently activate cell signaling pathways, collectively known as the Unfolded Protein Response (UPR). IRE1alpha is a central component of the UPR. In pancreatic beta-cells, IRE1alpha also functions in the regulation of insulin biosynthesis.
Principal findings: Here we report that hyperactivation of IRE1alpha caused by chronic high glucose treatment or IRE1alpha overexpression leads to insulin mRNA degradation in pancreatic beta-cells. Inhibition of IRE1alpha signaling using its dominant negative form prevents insulin mRNA degradation. Islets from mice heterozygous for IRE1alpha retain expression of more insulin mRNA after chronic high glucose treatment than do their wild-type littermates.
Conclusions/significance: These results reveal a role of IRE1alpha in insulin mRNA expression under ER stress conditions caused by chronic high glucose. The rapid degradation of insulin mRNA could provide immediate relief for the ER and free up the translocation machinery. Thus, this mechanism would preserve ER homeostasis and help ensure that the insulin already inside the ER can be properly folded and secreted. This adaptation may be crucial for the maintenance of beta-cell homeostasis and may explain why the beta-cells of type 2 diabetic patients with chronic hyperglycemia stop producing insulin in the absence of apoptosis. This mechanism may also be involved in suppression of the autoimmune type 1 diabetes by reducing the amount of misfolded insulin, which could be a source of "neo-autoantigens."
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
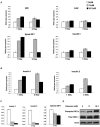
Figure 1. Chronic high-glucose treatment causes ER stress in islets and insulinoma cells, resulting in a reduction in insulin gene expression.
(A) Islets pooled from 6 mice were treated with 5 mM, 11 mM, or 16.7 mM glucose for 24 or 72 hr. Expression levels of Ero1α, Chop, spliced Xbp-1, and total Xbp-1 were measured by real- time PCR (n = 2). (B) Islets pooled from 6 mice were treated with 5 mM, 11 mM, or 16.7 mM glucose for 24 or 72 hr. Expression levels of Insulin 1 and Insulin 2 were measured by real time PCR (n = 2). (C) INS-1 832/13 cells were pretreated for 12 hr with 5 mM glucose, then treated with 5 mM, 11 mM, or 16.7 mM glucose for 72 hr. Expression levels of Insulin 1, Insulin 2, and spliced Xbp-1 were measured by real time PCR (n = 3; values are mean±SEM). (D) INS-1 832/13 cells were pretreated for 12 hr with 5 mM glucose, then treated with 5 mM, 11 mM, or 16.7 mM glucose for 72 hr. Total IRE1α, phosphorylated IRE1α, and actin were measured by immunoblot.
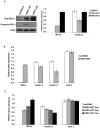
Figure 2. Overexpression of IRE1α correlates with reduced Insulin mRNA in cultured cells.
(A) COS-7 cells were transfected with mouse Insulin 2 and cultured for 24 hr. Cells were then split onto 3 plates and transfected again with wild-type human Ire1α; IRE1α WT, a kinase/endoribonuclease inactive mutant human Ire1α; IRE1α KA; or pcDNA3 control. They were then cultured for 24 hr. Protein and RNA were collected from the same plates. Total IRE1α, phosphorylated IRE1α, and actin were measured by immunoblot. Expression levels of human IRE1α and mouse Insulin 2 were measured by real time PCR (n = 3; values are mean±SEM). (B) INS-1 832/13 cells were transfected with human IRE1α WT or pcDNA3 control and cultured for 24 hr. Expression levels of human IRE1α, endogenous rat insulin 1, insulin 2, and glucose transporter 2 (glut 2) were measured by real time PCR (n = 3; values are mean±SEM). (C) INS-1 832/13 cells were transfected with either pcDNA3 control or increasing concentrations of human IRE1α WT and cultured for 24 hr. Expression levels of human IRE1α, endogenous rat insulin 1, insulin 2, and glucose transporter 2 (glut 2) were measured by real-time PCR (n = 3; values are mean±SEM).
Figure 3. Cells expressing mutant IRE1α resist both chemical- and glucose-induced Insulin mRNA degradation.
(A) pTetRINS-1 832/13 cells (control) and pTetRINS-1832/13IRE1αKA cells (stably expressing tetracycline-responsive K599A mutant IRE1α) were treated with doxycycline for 24 hr with 5 mM glucose to induce mutant IRE1α. Cells were then treated with 5 mM, 11 mM, or 16.7 mM glucose with doxycycline for 72 hr. Degradation of mRNA was assessed by measuring the expression of Insulin 1 and Insulin 2 by real-time PCR (n = 3; values are mean±SEM). (B) pTetRINS-1 832/13 cells (control) and pTetRINS-1832/13IRE1αKA cells (stably expressing tetracycline-responsive K599A mutant IRE1α) were treated with doxycycline for 24 hr to induce mutant IRE1α. mRNA transcription was attenuated by treating cells with 100 µg/mL actinomycin D for 1 hr. To induce degradation of insulin mRNA, 1 µM thapsigargin was added to the medium for 0, 1, 3 and 5 hr. mRNA degradation was assessed by measuring expression of Insulin 1 and Insulin 2 by real-time PCR (n = 3; values are mean±SEM).
Figure 4. IRE1α heterozygous islets resist the negative effects of chronic high glucose.
IRE1α WT or IRE1α heterozygous mouse islets were treated with 16.7 mM glucose for 72 hr. Expression of Insulin 1, Insulin 2, and spliced XBP-1 were measured by quantitative real-time PCR.
Similar articles
- [Pathogenic Mechanism of Diabetes Development Due to Dysfunction of Unfolded Protein Response].
Tsuchiya Y, Saito M, Kohno K. Tsuchiya Y, et al. Yakugaku Zasshi. 2016;136(6):817-25. doi: 10.1248/yakushi.15-00292-4. Yakugaku Zasshi. 2016. PMID: 27252061 Review. Japanese. - Nicotinic acetylcholine receptor signaling regulates inositol-requiring enzyme 1α activation to protect β-cells against terminal unfolded protein response under irremediable endoplasmic reticulum stress.
Ishibashi T, Morita S, Kishimoto S, Uraki S, Takeshima K, Furukawa Y, Inaba H, Ariyasu H, Iwakura H, Furuta H, Nishi M, Papa FR, Akamizu T. Ishibashi T, et al. J Diabetes Investig. 2020 Jul;11(4):801-813. doi: 10.1111/jdi.13211. Epub 2020 Feb 17. J Diabetes Investig. 2020. PMID: 31925927 Free PMC article. - ER stress and the decline and fall of pancreatic beta cells in type 1 diabetes.
Brozzi F, Eizirik DL. Brozzi F, et al. Ups J Med Sci. 2016 May;121(2):133-9. doi: 10.3109/03009734.2015.1135217. Epub 2016 Feb 22. Ups J Med Sci. 2016. PMID: 26899404 Free PMC article. Review. - Ubiquitin D Regulates IRE1α/c-Jun N-terminal Kinase (JNK) Protein-dependent Apoptosis in Pancreatic Beta Cells.
Brozzi F, Gerlo S, Grieco FA, Juusola M, Balhuizen A, Lievens S, Gysemans C, Bugliani M, Mathieu C, Marchetti P, Tavernier J, Eizirik DL. Brozzi F, et al. J Biol Chem. 2016 Jun 3;291(23):12040-56. doi: 10.1074/jbc.M115.704619. Epub 2016 Apr 4. J Biol Chem. 2016. PMID: 27044747 Free PMC article. - Pharmacological Inhibition of Inositol-Requiring Enzyme 1α RNase Activity Protects Pancreatic Beta Cell and Improves Diabetic Condition in Insulin Mutation-Induced Diabetes.
Herlea-Pana O, Eeda V, Undi RB, Lim HY, Wang W. Herlea-Pana O, et al. Front Endocrinol (Lausanne). 2021 Oct 5;12:749879. doi: 10.3389/fendo.2021.749879. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34675883 Free PMC article.
Cited by
- Wolfram Syndrome 1: A Neuropsychiatric Perspective on a Rare Disease.
Caruso V, Raia A, Rigoli L. Caruso V, et al. Genes (Basel). 2024 Jul 25;15(8):984. doi: 10.3390/genes15080984. Genes (Basel). 2024. PMID: 39202345 Free PMC article. Review. - Endoplasmic reticulum stress and therapeutic strategies in metabolic, neurodegenerative diseases and cancer.
Yuan S, She D, Jiang S, Deng N, Peng J, Ma L. Yuan S, et al. Mol Med. 2024 Mar 20;30(1):40. doi: 10.1186/s10020-024-00808-9. Mol Med. 2024. PMID: 38509524 Free PMC article. Review. - IRE1 RNase controls CD95-mediated cell death.
Pelizzari-Raymundo D, Maltret V, Nivet M, Pineau R, Papaioannou A, Zhou X, Caradec F, Martin S, Le Gallo M, Avril T, Chevet E, Lafont E. Pelizzari-Raymundo D, et al. EMBO Rep. 2024 Apr;25(4):1792-1813. doi: 10.1038/s44319-024-00095-9. Epub 2024 Feb 21. EMBO Rep. 2024. PMID: 38383861 Free PMC article. - UBXN1 maintains ER proteostasis and represses UPR activation by modulating translation.
Ahlstedt BA, Ganji R, Mukkavalli S, Paulo JA, Gygi SP, Raman M. Ahlstedt BA, et al. EMBO Rep. 2024 Feb;25(2):672-703. doi: 10.1038/s44319-023-00027-z. Epub 2024 Jan 2. EMBO Rep. 2024. PMID: 38177917 Free PMC article.
References
- Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. - PubMed
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
- Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci 2007 - PubMed
- Aridor M, Balch WE. Integration of endoplasmic reticulum signaling in health and disease. Nat Med. 1999;5:745–751. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials