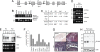The stumpy gene is required for mammalian ciliogenesis - PubMed (original) (raw)
Comparative Study
. 2008 Feb 26;105(8):2853-8.
doi: 10.1073/pnas.0712385105. Epub 2008 Feb 19.
Affiliations
- PMID: 18287022
- PMCID: PMC2268549
- DOI: 10.1073/pnas.0712385105
Comparative Study
The stumpy gene is required for mammalian ciliogenesis
Terrence Town et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Cilia are present on nearly all cell types in mammals and perform remarkably diverse functions. However, the mechanisms underlying ciliogenesis are unclear. Here, we cloned a previously uncharacterized highly conserved gene, stumpy, located on mouse chromosome 7. Stumpy was ubiquitously expressed, and conditional loss in mouse resulted in complete penetrance of perinatal hydrocephalus (HC) and severe polycystic kidney disease (PKD). We found that cilia in stumpy mutant brain and kidney cells were absent or markedly deformed, resulting in defective flow of cerebrospinal fluid. Stumpy colocalized with ciliary basal bodies, physically interacted with gamma-tubulin, and was present along ciliary axonemes, suggesting that stumpy plays a role in ciliary axoneme extension. Therefore, stumpy is essential for ciliogenesis and may be involved in the pathogenesis of human congenital malformations such as HC and PKD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Generation of stumpy mutant mice and gene expression analysis. (A) Diagram showing insertion of loxP sites flanking exon 4 of stumpy and exon 1 of tgfb1. Positions of primers a–d used for RT-PCR or Northern blot probe are shown. (B) Multiplex PCR designed to detect floxed (but not deleted), deleted, or wild-type alleles at P0 from floxed but not deleted (+/+) or homozygous deleted (fl/fl) mice. (C) Affymetrix GeneChip results showing mRNA expression of stumpy or tgfb1 in P0 and P12 brains from fl/fl vs. heterozygous (+/fl) mice. (D) RT-PCR using primer pairs shown in A. (E) Northern blot using a probe amplified from primers c and d shown in A reveals tgfb1 or a chimeric stumpy-tgfb1 in-frame read-through transcript in brains from fl/fl vs. +/fl mice. (F) Q-PCR analysis of stumpy relative to hprt1 in mouse organs and cells. (G) RNA in situ hybridization with stumpy α-sense or sense (control) probes in wild-type C57BL/6 mouse brains. Higher-magnification images (Lower, scale bar denotes 50 μm, calculated for Lower Left and Lower Right) reveal expression in cells lining the 4v, the S. aq, Lv, and in C. Plexus (cp). (H) Brain homogenates from P12 +/+ or fl/fl mice were Western blotted with stumpy antibody (Upper). Note the ≈19-kDa band corresponding to the predicted molecular mass for stumpy. * shows a possible stumpy degraded product, and actin is shown Lower as a loading control.
Fig. 2.
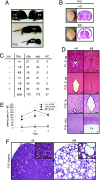
PTvHC communicating HC and polycystic kidney disease in stumpy mutant mice. (A) Photographs of representative +fl and fl/fl mice (Upper) showing classic “domed head” of hydrocephalic fl/fl mice at P16. By P12, fl/fl mice consistently appear runted (Lower). (B) Whole brains isolated from +/fl and fl/fl mice (Left), and low-magnification photomicrographs of H&E-stained coronal brain sections (Right) showing pronounced venticulomegaly. (C) _Nestin-cre_-positive mice bearing one floxed allele (cre +, +/fl) were crossed with _nestin-cre_-negative –, +/fl mice, and all deficient offspring (cre +, fl/fl) manifested perinatal hydrocephaly. An additional 20/104 hydrocephalic cre +, fl/fl mice were identified from cre +, +/fl x cre−, fl/fl breedings. (D) Coronal H&E-stained sections show complete stenosis of the S. aq in fl/fl brains (Right). An enlarged 3v and thinner neocortex (nctx) are also observed in fl/fl brains (Right). (Scale bars, 100 μm.) (E) Motor function in wild-type (+/+, n = 9), +/fl (n = 10), and fl/fl mice (n = 6) was assayed by accelerating roto-rod, and data are represented as means ± SE of time to fall (latency, in seconds) for each of three consecutive trials. *, P < 0.001 for fl/fl vs. +/fl or +/+. (F) H&E-stained sections from +/fl or fl/fl P12 kidneys show evidence of polycystic kidney disease. High-magnification Insets are shown in the upper right corners. (Scale bars, 20 μm.)
Fig. 3.
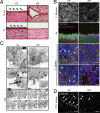
Ciliogenesis defects in stumpy mutant mice. (A) P16 +/fl or fl/fl H&E-stained sections showing the S. aq (Upper) and the ependyma of 3v (Lower). Arrowheads indicate cilia. (Scale bars, 20 μm.) (B) Vibratome brain sections from the F. Monroe (Top) or cryosections from 4v or the C. Plexus from +/fl (Left) or fl/fl mice (Right) at P4 were immunostained for acetylated α-tubulin (against the ciliary axoneme), rootletin (against ciliary rootlets), and/or γ-tubulin (recognizes ciliary bb) (colors for each antibody are indicated), and nuclear counterstained with DAPI (blue). Images were captured by using a Zeiss ApoTome-equipped fluorescence microscope (Top) or a confocal microscope (all other images). Arrowheads in the C. Plexus show ciliary axonemes. (Scale bars, 10 μm.) (C) Transmission electron micrographs of cells lining the S. aq are shown from P0 +/fl (Left) or fl/fl mice (Right and Bottom). Higher magnifications of Insets (Top) are shown below in Middle (Middle Insets show additional examples). Note the presence of bb structures in both +/fl and fl/fl mice, whereas fl/fl cells either do not have ciliary axonemes (c) or they appear deformed. Bottom show serial EM sections (0–420 nm) through bb structures of the boxed region, demonstrating the absence of c structures in a mutant S. aq cell. [Scale bars: Top, 2 μm; Middle and Bottom, 0.5 μm.] (D) Confocal microscopy was performed on kidneys isolated from P12 +/fl or fl/fl mice after α-tubulin immunostaining. Arrowheads indicate cilia. (Scale bars, 20 μm.)
Fig. 4.
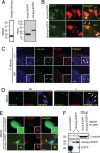
Stumpy is an essential component of the ciliary bb and axoneme. (A) MDCK (Left) or HeLa (Right) cells were transiently transfected with stumpy-his/myc, mock-EGFP, or stumpy-EGFP plasmids. After 48 h, cell lysates were Western blotted with anti-myc or GFP antibodies. (B) Embryonic day 15 fetuses were coelectroporated with plasmids encoding RFP and mock-EGFP or stumpy-EGFP. RFP+ ependymal cells along Lv were imaged at P10. Arrowheads show apical localization of _stumpy_-EGFP compared with diffuse EGFP signal in Mock-transfected cells. (Scale bar, 5 μm.) (C) C. Plexus from P8 +/+ or fl/fl mice was immunostained for γ-tubulin (green signal) and stumpy (red signal) (arrowheads show colocalization; high-magnification Insets are in the bottom right). (Scale bar, 20 μm.) (D) Differentiated MDCK cells were immunostained for stumpy (green signal) or α-tubulin (red signal) (merged images are shown to the right). (Scale bar, 5 μm.) Note stumpy signal at the bb (Left, see arrowhead) and ciliary axoneme (c) (Right; see arrowheads). MDCK cells were stably transfected with mock-EGFP or stumpy-EGFP plasmids (green signal), and (E) immunostained with γ-tubulin (red signal) (merged images are shown to the right; arrowhead shows colocalized signals; high-magnification Insets are in the bottom right) (scale bar, 5 μm) or (F) cell lysates were immunoprecipitated with GFP antibody and Western blotted with γ-tubulin (Upper) or GFP antibody (Lower). Ten percent input controls are shown to the right, and * indicates rabbit Ig heavy chain. For C–E, DAPI was used as a nuclear counterstain (blue signal). Images were captured by using a confocal microscope (B and C), a Zeiss ApoTome-equipped fluorescence microscope (D), or an epifluorescence microscope (E).
Similar articles
- SNX27 Deletion Causes Hydrocephalus by Impairing Ependymal Cell Differentiation and Ciliogenesis.
Wang X, Zhou Y, Wang J, Tseng IC, Huang T, Zhao Y, Zheng Q, Gao Y, Luo H, Zhang X, Bu G, Hong W, Xu H. Wang X, et al. J Neurosci. 2016 Dec 14;36(50):12586-12597. doi: 10.1523/JNEUROSCI.1620-16.2016. J Neurosci. 2016. PMID: 27974614 Free PMC article. - Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease.
Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Lin F, et al. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5286-91. doi: 10.1073/pnas.0836980100. Epub 2003 Apr 2. Proc Natl Acad Sci U S A. 2003. PMID: 12672950 Free PMC article. - Ulk4 Is Essential for Ciliogenesis and CSF Flow.
Liu M, Guan Z, Shen Q, Lalor P, Fitzgerald U, O'Brien T, Dockery P, Shen S. Liu M, et al. J Neurosci. 2016 Jul 20;36(29):7589-600. doi: 10.1523/JNEUROSCI.0621-16.2016. J Neurosci. 2016. PMID: 27445138 Free PMC article. - Ciliopathies and the Kidney: A Review.
McConnachie DJ, Stow JL, Mallett AJ. McConnachie DJ, et al. Am J Kidney Dis. 2021 Mar;77(3):410-419. doi: 10.1053/j.ajkd.2020.08.012. Epub 2020 Oct 9. Am J Kidney Dis. 2021. PMID: 33039432 Review. - Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia.
Lee L. Lee L. J Neurosci Res. 2013 Sep;91(9):1117-32. doi: 10.1002/jnr.23238. Epub 2013 May 17. J Neurosci Res. 2013. PMID: 23686703 Review.
Cited by
- Disruption of the mouse Jhy gene causes abnormal ciliary microtubule patterning and juvenile hydrocephalus.
Appelbe OK, Bollman B, Attarwala A, Triebes LA, Muniz-Talavera H, Curry DJ, Schmidt JV. Appelbe OK, et al. Dev Biol. 2013 Oct 1;382(1):172-85. doi: 10.1016/j.ydbio.2013.07.003. Epub 2013 Jul 29. Dev Biol. 2013. PMID: 23906841 Free PMC article. - New functions of B9D2 in tight junctions and epithelial polarity.
Caenen-Braz C, Bouzhir L, Dupuis-Williams P. Caenen-Braz C, et al. Sci Rep. 2024 Oct 25;14(1):25293. doi: 10.1038/s41598-024-75577-w. Sci Rep. 2024. PMID: 39455645 Free PMC article. - The primary cilium at the crossroads of mammalian hedgehog signaling.
Wong SY, Reiter JF. Wong SY, et al. Curr Top Dev Biol. 2008;85:225-60. doi: 10.1016/S0070-2153(08)00809-0. Curr Top Dev Biol. 2008. PMID: 19147008 Free PMC article. Review. - A novel hypomorphic allele of Spag17 causes primary ciliary dyskinesia phenotypes in mice.
Abdelhamed Z, Lukacs M, Cindric S, Ali S, Omran H, Stottmann RW. Abdelhamed Z, et al. Dis Model Mech. 2020 Oct 30;13(10):dmm045344. doi: 10.1242/dmm.045344. Dis Model Mech. 2020. PMID: 32988999 Free PMC article. - Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus.
Abouhamed M, Grobe K, San IV, Thelen S, Honnert U, Balda MS, Matter K, Bähler M. Abouhamed M, et al. Mol Biol Cell. 2009 Dec;20(24):5074-85. doi: 10.1091/mbc.e09-04-0291. Mol Biol Cell. 2009. PMID: 19828736 Free PMC article.
References
- Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol. 2005;289:F1159–F1169. - PubMed
- Ibanez-Tallon I, et al. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum Mol Genet. 2004;13:2133–2141. - PubMed
- Banizs B, et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132:5329–5339. - PubMed
- Sawamoto K, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases