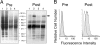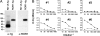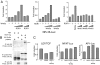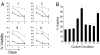Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a - PubMed (original) (raw)
Comparative Study
. 2008 Feb 26;105(8):3047-52.
doi: 10.1073/pnas.0712148105. Epub 2008 Feb 19.
Liguang Chen, Tomoyuki Endo, Li Tang, Desheng Lu, Januario E Castro, George F Widhopf 2nd, Laura Z Rassenti, Mark J Cantwell, Charles E Prussak, Dennis A Carson, Thomas J Kipps
Affiliations
- PMID: 18287027
- PMCID: PMC2268582
- DOI: 10.1073/pnas.0712148105
Comparative Study
Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a
Tetsuya Fukuda et al. Proc Natl Acad Sci U S A. 2008.
Abstract
We examined the sera of six patients before and after i.v. infusions of autologous chronic lymphocytic leukemia (CLL) cells transduced ex vivo with an adenovirus encoding CD154 (Ad-CD154). Five patients made high-titer antibodies against adenovirus and three made IgG reactive with a leukemia-associated surface antigen, which we identified as ROR1. Anti-ROR1 antibodies were not detected in the sera of untreated patients. We generated anti-ROR1 mAbs and found they reacted specifically with the CLL cells of all patients, but not with nonleukemic leukocytes, a wide variety of normal adult tissues, or blood mononuclear cells, including CD5(+) B cells of healthy adults. ROR1 could bind Wnt5a, which induced activation of NF-kappaB when coexpressed with ROR1 in HEK293 cells and enhanced the survival of CLL cells in vitro, an effect that could be neutralized by posttreatment anti-ROR1 antisera. We conclude that patients with CLL can break immune tolerance to ROR1, which is an oncofetal surface antigen and survival-signaling receptor in this neoplastic disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Evaluation for IgG Anti-CLL antibodies. (A) Lysates of membrane proteins isolated from CHO cells (lane 1), CHO-ROR1 cells (lane 2), lymphocytes of a healthy donor (lane 3), or the CLL cells of an untreated patient (lane 4) were examined by immunoblot analysis with sera obtained before (Left) or after (Right) infusions of autologous, Ad-CD154-CLL cells. The blots were treated with HRP-conjugated mouse anti-human IgG and developed with chemiluminescent substrate for autoradiography. The numbers indicate the molecular sizes of marker proteins that were electrophoresed in parallel lanes. The arrow highlights the band of ≈125 kDa identified in the lysates of CHO-ROR1 cells (lane 3) or CLL cells (lane 4) by using the postinfusion sera (Right). (B) The histograms depict the reactivity of sera with CHO cells (open histograms), or CHO-ROR1 cells (shaded histograms) before (Left) or after (Right) infusion. After washing the cells, the cell-bound antibody was detected by using a fluorochrome-conjugated goat anti-human Ig.
Fig. 2.
Anti-ROR1 antibody detected by ELISA. (A) Immunoblots of ROR1-rIg or control K8.1A-rig that subsequently were probed with goat anti-rabbit Ig (α rig) (Left) or goat anti-ROR1 Ig (α ROR1) (Right). The lanes containing the K8.1A-rig or ROR1-rig are indicated. The isolated proteins were assessed for purity by using GelCode blue stain reagent (Pierce) staining after the SDS/PAGE. The numbers indicate the molecular sizes of marker proteins run in parallel lanes of the SDS/PAGE. (B) ELISA for anti-ROR1 antibodies in sera from each patient. The open circles connected with dashed lines indicate the data with pretreatment sera, and the closed squares connected by lines indicate the data of posttreatment sera. The optical density (at 450 nm) is indicated for the serum samples at each of various dilutions (as indicated by the inverse of the dilution factor on the x axis).
Fig. 3.
Reactivity of 4A5 with CLL cells and nonneoplastic lymphocytes. (A) Histograms depicting the fluorescence of CLL cells stained with 4A5 (shaded histograms) or an IgG2b isotype control antibody of irrelevant specificity (open histograms). Depicted is a typical histogram for stained CLL cells that express ZAP-70 (Upper) or that lacked expression of ZAP-70 (Lower), that was detected, as described (35). (B) The peripheral blood mononuclear cells (PBMC) of a healthy adult were stained as in Fig. 4_A_ using 4A5 or the control IgG2b along with fluorochrome-conjugated mAb specific for CD5, and CD19. The expression of CD5 (y axis) and CD19 (x axis) by the stained PBMC is depicted by using a 2D contour plot (Lower Left). The 4A5 staining of the PBMC (Upper Left), the CD5+/CD19+ B cells (Upper Right), or the total CD19+ cells (Lower Right) is provided by using gating strategies as indicated in the contour plot. The arrows lead from the electronically gated subpopulation of CD5+/CD19+B cells to the representative histogram. The shaded histograms depict the fluorescence of cells stained with 4A5 and the open histograms provide the fluorescence of cells stained with the IgG2b isotype control mAb.
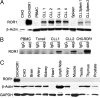
Fig. 4.
Immunoblot analyses for expression of ROR1. (A) Total cell lysates of CHO cells, CHO-ROR1 cells, CLL blood mononuclear cells (CLL samples 1–4), or CLL splenocytes (CLL spleen 1 and CLL spleen 2), blood mononuclear cells of a healthy donor (PBMC), or nonneoplastic, normal human splenocytes (Spleen) were examined by immunoblot analysis using rabbit anti-ROR1 anti-peptide antibody (Upper) or antibodies to β-actin to monitor for protein loading (Lower). The source of the tissue is indicated at the top of each lane. (B) Immunoprecipitation of ROR1 using the 4A5 mAb. Cell lysates of normal donor PBMC, normal tonsil, CLL blood mononuclear cells (CLL1 and CLL2) or CHO-ROR1 cells were incubated with the 4A5 mAb or an IgG isotype control mAb for immune precipitation using Staph protein A. The immune precipitate was evaluated by immunoblot analysis using anti-ROR1 peptide antisera. This process detected protein of ≈125 kDa in 4A5 immune precipitates prepared from CLL cell samples or CHO-ROR1 cells, but not from blood or tonsillar lymphocytes of normal donors, or the isotype control immune precipitates from any source. (C) Cells lysates were prepared from CHO-ROR1 cells, CHO cells, or human brain, artery, kidney, heart, lymph node, ovary, skeletal muscle, testis, thymus, or prostate tissue for immunoblot analyses using anti-ROR1 antibodies (Top) or antibodies specific for β-actin (Middle) or GADPH (Bottom), to monitor for protein loading. Antibodies were used to monitor for GADPH because not all tissues have detectable β-actin in this assay (e.g., cardiac and skeletal muscle).
Fig. 5.
Functional studies on ROR1. (A) Effect of ROR1 on NF-κB activity. HEK293 cells were cotransduced with NF-κB reporter construct and β-gal vector along with expression vectors encoding ROR1, Wnt5a, Wnt3, Wnt5b, or Wnt16. (Left) Shown are the data for NF-κB activation of cells transduced without (−) or with (+) the vector encoding Wnt5a and increasing amounts of vector encoding ROR1. (Middle) Shown are the data for NF-κB activation of cells transduced without (−) or with (+) the vector encoding ROR1 and increasing amounts of vector encoding Wnt5a. (Right) Shown are the data for NF-κB activation of cells transduced without (−) or with (+) the vector encoding ROR1 and the vector encoding Wnt5a or increasing amounts of vector encoding Wnt3, Wnt5b, or Wnt16, respectively. Each bar provides the mean fold induction over baseline of triplicate test samples ± SE. (B) In vitro binding of ROR1 to Wnt5a. Cells were transduced with vectors encoding an HA-tagged Wnt5a recombinant protein (designated HA-Wnt5a). The conditioned media of such cells were incubated with or without ROR1-rIg or rIgG before immune precipitation using anti-HA antibodies (Top and Bottom) or protein-A/G (Middle). The blots were probed with antibodies specific for rIgG (Top and Middle) or anti-HA (Bottom). At the top of each lane is the combination of reagents used in the immune precipitation studies. + indicates that the constituent designated on the left was used in preparing the sample for that specific lane. The lanes marked with + for pcDNA3 used conditioned supernatant from cells transduced with control vector that did not encode Wnt5a or ROR1. (C) Effect of ROR1 on lymphoid enhancer-binding factor/T cell factor (LEF/TCF)-sensitive luciferase reporter gene TOPFLASH (Left), nuclear factor of activated T cells (NF-AT) (Center), or activator protein 1 (AP-1) (Right). HEK293 cells were cotransduced with indicated reporter constructs, a vector encoding β-gal (control labeled C), or vectors encoding ROR1 and/or Wnt5a. Each bar provides the mean fold induction over baseline of triplicate test samples ± SE.
Fig. 6.
Influence of Wnt5a on CLL cell viability. (A) Effect of Wnt5a on the viability of CLL cells cultured in vitro. CLL cells from each of four unrelated patients, designated CLL 1, CLL 2, CLL 3, and CLL 4, were cultured alone (■) or together with CHO cells (♦), or CHO-Wnt5a cells (○). The percent viability of the CD19+ CLL cells, indicated on the ordinate, was assessed by flow cytometry on days 1, 2, and 3 of culture. Each data point represents the mean value of quadruplicate samples cultured in parallel ± SE. (B) CLL cells were cultured for 2 days in RPMI medium 1640 containing 20% human serum, either alone or together with CHO cells or CHO-Wnt5a cells and then assessed for viability by flow cytometry. The bars indicate the mean percent viability of the CD19+ CLL cells of quadrulicate wells for each culture condition. Except for condition 1, all cultures had serum samples from patient 4 collected either before or 2 weeks after the last infusion of autologous Ad-CD154-trasduced CLL cells. CLL cells were cultured alone in media containing normal human serum (condition 1), preinfusion serum (condition 2), or postinfusion serum (condition 3). For culture conditions 4, 6, and 8 the CLL cells were cocultured with CHO cells and for culture conditions 5, 7, and 9 the CLL cells were cocultured with CHO-Wnt5a cells. Cultures 4 and 5 used preinfusion serum, cultures 6 and 7 used postinfusion serum that had been absorbed with CHO cells, and cultures 8 and 9 used postinfusion serum that had been absorbed with CHO-ROR1 cells to remove anti-ROR1 binding activity. The error bars depict the SE about the mean of quadruplicate wells cultured in parallel. The asterisks indicate that the viability of CLL cells in those culture condition was significantly greater than that of the other culture conditions by Bonferroni t test (P < 0.05).
Similar articles
- Wnt5a induces ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and proliferation.
Yu J, Chen L, Cui B, Widhopf GF 2nd, Shen Z, Wu R, Zhang L, Zhang S, Briggs SP, Kipps TJ. Yu J, et al. J Clin Invest. 2016 Feb;126(2):585-98. doi: 10.1172/JCI83535. J Clin Invest. 2016. PMID: 26690702 Free PMC article. - Autocrine Signaling by Wnt-5a Deregulates Chemotaxis of Leukemic Cells and Predicts Clinical Outcome in Chronic Lymphocytic Leukemia.
Janovska P, Poppova L, Plevova K, Plesingerova H, Behal M, Kaucka M, Ovesna P, Hlozkova M, Borsky M, Stehlikova O, Brychtova Y, Doubek M, Machalova M, Baskar S, Kozubik A, Pospisilova S, Pavlova S, Bryja V. Janovska P, et al. Clin Cancer Res. 2016 Jan 15;22(2):459-69. doi: 10.1158/1078-0432.CCR-15-0154. Epub 2015 Aug 3. Clin Cancer Res. 2016. PMID: 26240275 Free PMC article. - Cirmtuzumab blocks Wnt5a/ROR1 stimulation of NF-κB to repress autocrine STAT3 activation in chronic lymphocytic leukemia.
Chen Y, Chen L, Yu J, Ghia EM, Choi MY, Zhang L, Zhang S, Sanchez-Lopez E, Widhopf GF 2nd, Messer K, Rassenti LZ, Jamieson C, Kipps TJ. Chen Y, et al. Blood. 2019 Sep 26;134(13):1084-1094. doi: 10.1182/blood.2019001366. Epub 2019 Aug 13. Blood. 2019. PMID: 31409670 Free PMC article. - Pre-clinical Specificity and Safety of UC-961, a First-In-Class Monoclonal Antibody Targeting ROR1.
Choi MY, Widhopf GF 2nd, Wu CC, Cui B, Lao F, Sadarangani A, Cavagnaro J, Prussak C, Carson DA, Jamieson C, Kipps TJ. Choi MY, et al. Clin Lymphoma Myeloma Leuk. 2015 Jun;15 Suppl(0):S167-9. doi: 10.1016/j.clml.2015.02.010. Clin Lymphoma Myeloma Leuk. 2015. PMID: 26297272 Free PMC article. Review. - The receptor tyrosine kinase ROR1--an oncofetal antigen for targeted cancer therapy.
Hojjat-Farsangi M, Moshfegh A, Daneshmanesh AH, Khan AS, Mikaelsson E, Osterborg A, Mellstedt H. Hojjat-Farsangi M, et al. Semin Cancer Biol. 2014 Dec;29:21-31. doi: 10.1016/j.semcancer.2014.07.005. Epub 2014 Jul 25. Semin Cancer Biol. 2014. PMID: 25068995 Review.
Cited by
- High expression level of ROR1 and ROR1-signaling associates with venetoclax resistance in chronic lymphocytic leukemia.
Ghia EM, Rassenti LZ, Choi MY, Quijada-Álamo M, Chu E, Widhopf GF 2nd, Kipps TJ. Ghia EM, et al. Leukemia. 2022 Jun;36(6):1609-1618. doi: 10.1038/s41375-022-01543-y. Epub 2022 Apr 13. Leukemia. 2022. PMID: 35418613 Free PMC article. - Selective depletion of a minor subpopulation of B-chronic lymphocytic leukemia cells is followed by a delayed but progressive loss of bulk tumor cells and disease regression.
Foster AE, Okur FV, Biagi E, Lu A, Dotti G, Yvon E, Savoldo B, Carrum G, Andreeff M, Goodell MA, Heslop HE, Brenner MK. Foster AE, et al. Mol Cancer. 2009 Nov 18;8:106. doi: 10.1186/1476-4598-8-106. Mol Cancer. 2009. PMID: 19922650 Free PMC article. - Spiperone enhances intracellular calcium level and inhibits the Wnt signaling pathway.
Lu D, Carson DA. Lu D, et al. BMC Pharmacol. 2009 Nov 30;9:13. doi: 10.1186/1471-2210-9-13. BMC Pharmacol. 2009. PMID: 19948059 Free PMC article. - Molecular, functional, and gene expression analysis of zebrafish Ror1 receptor.
Bai Y, Liu C, Zhou J, Rong X, Wang H. Bai Y, et al. Fish Physiol Biochem. 2019 Feb;45(1):355-363. doi: 10.1007/s10695-018-0567-0. Epub 2018 Sep 21. Fish Physiol Biochem. 2019. PMID: 30242697 - Prospects for pharmacological targeting of pseudokinases.
Kung JE, Jura N. Kung JE, et al. Nat Rev Drug Discov. 2019 Jul;18(7):501-526. doi: 10.1038/s41573-019-0018-3. Nat Rev Drug Discov. 2019. PMID: 30850748 Free PMC article. Review.
References
- Sinisalo M, et al. Response to vaccination against different types of antigens in patients with chronic lymphocytic leukemia. Br J Haematol. 2001;114:107–110. - PubMed
- van der Velden AM, et al. Influenza virus vaccination and booster in B-cell chronic lymphocytic leukaemia patients. Eur J Intern Med. 2001;12:420–424. - PubMed
- Hartkamp A, Mulder AH, Rijkers GT, van Velzen-Blad H, Biesma DH. Antibody responses to pneumococcal and hemophilus vaccinations in patients with B-cell chronic lymphocytic leukemia. Vaccine. 2001;19:1671–1677. - PubMed
- Ranheim EA, Cantwell MJ, Kipps TJ. Expression of CD27 and its ligand, CD70, on chronic lymphocytic leukemia B cells. Blood. 1995;85:3556–3565. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases