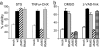Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein - PubMed (original) (raw)
Comparative Study
. 2008 Feb 26;105(8):3094-9.
doi: 10.1073/pnas.0800168105. Epub 2008 Feb 19.
Affiliations
- PMID: 18287053
- PMCID: PMC2268590
- DOI: 10.1073/pnas.0800168105
Comparative Study
Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein
Claudia Mack et al. Proc Natl Acad Sci U S A. 2008.
Abstract
TNFalpha is an important cytokine in antimicrobial immunity and inflammation. The receptor-interacting protein RIP1 is an essential component of the TNF receptor 1 signaling pathway that mediates the activation of NF-kappaB, MAPKs, and programmed cell death. It also transduces signals derived from Toll-like receptors and intracellular sensors of DNA damage and double-stranded RNA. Here, we show that the murine CMV M45 protein binds to RIP1 and inhibits TNFalpha-induced activation of NF-kappaB, p38 MAPK, and caspase-independent cell death. M45 also inhibited NF-kappaB activation upon stimulation of Toll-like receptor 3 and ubiquitination of RIP1, which is required for NF-kappaB activation. Hence, M45 functions as a viral inhibitor of RIP1-mediated signaling. The results presented here reveal a mechanism of viral immune subversion and demonstrate how a viral protein can simultaneously block proinflammatory and innate immune signaling pathways by interacting with a central mediator molecule.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
M45 is required to block TNFα-induced caspase-independent cell death. (a) 10.1 fibroblasts were infected with MCMVs as indicated and treated 6 h later with STS or TNFα plus CHX. Viability was measured 20 h later relative to cells treated with solvent only (for STS) or CHX only (for TNFα). Results that were similar to those with TNFα plus CHX were obtained with TNFα alone, with the exception of mock-infected cells, which did not die from TNFα alone (data not shown). (b) Cells were infected and treated with TNFα plus CHX and the caspase inhibitor z-VAD-fmk or DMSO. Cells infected with ΔM45 or ΔM36 were sensitive to TNFα-induced cell death, which was blocked by z-VAD-fmk only in the case of the ΔM36 virus.
Fig. 2.
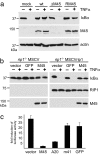
M45 binds to RIP1. (a) Lysates of cells infected with wt MCMV or an HA-tagged virus (M45HA) were used for immunoprecipitation with the indicated antibodies. RIP1 was detected by Western blot as a protein coprecipitating with M45. (b) HA-tagged M45 was coprecipitated with RIP1 in lysates of cells infected with MCMV–M45HA. (c) NIH 3T3 cells were transfected with plasmids expressing HA-tagged M45 or m143 (a different MCMV protein), respectively. RIP1 was coprecipitated with M45 but not with m143. (d) 3T3 fibroblasts derived from wt and knockout mice were infected with wt or M45HA-expressing MCMV. RIP1 was coprecipitated with M45HA in all but _rip1_−/− knockout cells.
Fig. 3.
M45 inhibits TNFα-induced NF-κB activation. (a) NIH 3T3 cells were infected with wt or mutant MCMV for 24 h and treated with TNFα for 15 min. M45-expressing viruses inhibited the degradation of IκBα. (b) _rip1_−/− knockout fibroblasts were stably transduced with a RIP1-expressing or an empty MSCV retrovirus. Cells were also transduced with retroviral vectors expressing M45 or GFP (as control). TNFα-induced IκBα degradation was blocked in all RIP1-deficient cells and in RIP1-positive cells expressing M45. (c) HEK 293 cells were transfected with an NF-κB-dependent luciferase reporter plasmid and different expression plasmids. Luciferase activity was measured 12 h after TNFα stimulation and is shown as fold induction compared with transfected cells without TNFα. Luciferase induction was inhibited in cells expressing M45 or the cellular RIP1 inhibitor A20 but not in cells transfected with control plasmids expressing irrelevant proteins (m41 or GFP) or empty pcDNA3 vector.
Fig. 4.
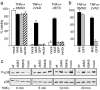
M45 inhibits caspase-independent cell death and activation of p38 MAPK. (a) SVEC4–10 endothelial cells were transduced with retroviral vectors expressing M45, GFP, or nothing. Cells were treated with TNFα (without CHX) and z-VAD-fmk, z-IETD-fmk, or DMSO. Viability was measured after 24 h. M45 blocked the induction of caspase-independent cell death. (b) Similar results were obtained with L929 fibrosarcoma cells. (c) Fibroblasts were infected with wt or mutant MCMV and treated with TNFα for the indicated periods of time. Lower amounts of phosphorylated p38 MAPK (P-p38) were detected in cells infected with M45-expressing viruses (wt and RM45).
Fig. 5.
Large parts of the N-terminal, but not of the C-terminal, domain of M45 are dispensable for interaction with RIP1 and activation of NF-κB. (a) Schematic representation of M45 and truncation mutants. The unique N-terminal domain is shown as an open box, and the C-terminal HA tag is shown in black. (b) NIH 3T3 cells were transfected with plasmids expressing full-length or truncated M45, and HA-tagged proteins were immunoprecipitated. RIP1 was coprecipitated with Nt1, -2, and -3 but not with Ct. (c) Luciferase activity was measured in transfected HEK 293 cells as described in the legend of Fig. 3_c_. The N-terminal, but not the C-terminal, truncation mutants inhibited TNFα-induced NF-κB activation.
Fig. 6.
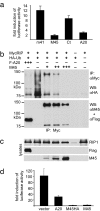
M45 inhibits TLR3-induced NF-κB activation and ubiquitination of RIP1. (a) HEK 293 cells were transfected with an NF-κB-dependent luciferase reporter plasmid and expression plasmids for TLR3 and the indicated proteins. Luciferase activity was measured 6 h after poly(I:C) addition and is shown as a fold induction as compared with transfected cells without poly(I:C). Induction of luciferase activity was inhibited by M45 and A20 but not by an irrelevant MCMV protein (m41) or a truncated M45 (Ct). (b) (Upper) Cells were transfected with plasmids as indicated. The total amount of plasmid for each transfection was normalized with empty pcDNA3 vector. MycRIP was precipitated with an anti-Myc antibody, and polyubiquitinated RIP1 was detected as a high-molecular-weight ladder by using an anti-HA antibody (lane 2). Ubiquitination was reduced in cells expressing A20 or M45. (Lower) The same blot after stripping and incubation with anti-M45 and anti-Flag antibodies. Comparing the two blots reveals that the anti-HA antibody also detects coprecipitated M45 and A20, indicating that A20 and M45 are also ubiquitinated. (c) Control Western blots show MycRIP, Flag-A20, and M45 in the cell lysates used for immunoprecipitation. (d) Overexpression of MycRIP in HEK 293 cells activates NF-κB, as measured with a luciferase reporter assay. NF-κB activation is inhibited by M45 and A20.
Similar articles
- Murine cytomegalovirus virion-associated protein M45 mediates rapid NF-κB activation after infection.
Krause E, de Graaf M, Fliss PM, Dölken L, Brune W. Krause E, et al. J Virol. 2014 Sep 1;88(17):9963-75. doi: 10.1128/JVI.00684-14. Epub 2014 Jun 18. J Virol. 2014. PMID: 24942588 Free PMC article. - RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition.
Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, Mocarski ES. Kaiser WJ, et al. Proc Natl Acad Sci U S A. 2014 May 27;111(21):7753-8. doi: 10.1073/pnas.1401857111. Epub 2014 May 12. Proc Natl Acad Sci U S A. 2014. PMID: 24821786 Free PMC article. - Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1.
Upton JW, Kaiser WJ, Mocarski ES. Upton JW, et al. J Biol Chem. 2008 Jun 20;283(25):16966-70. doi: 10.1074/jbc.C800051200. Epub 2008 Apr 28. J Biol Chem. 2008. PMID: 18442983 Free PMC article. - Mechanisms and pathways of innate immune activation and regulation in health and cancer.
Cui J, Chen Y, Wang HY, Wang RF. Cui J, et al. Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review. - Control of life-or-death decisions by RIP1 kinase.
Christofferson DE, Li Y, Yuan J. Christofferson DE, et al. Annu Rev Physiol. 2014;76:129-50. doi: 10.1146/annurev-physiol-021113-170259. Epub 2013 Sep 20. Annu Rev Physiol. 2014. PMID: 24079414 Review.
Cited by
- A cytomegalovirus inflammasome inhibitor reduces proinflammatory cytokine release and pyroptosis.
Deng Y, Ostermann E, Brune W. Deng Y, et al. Nat Commun. 2024 Jan 26;15(1):786. doi: 10.1038/s41467-024-45151-z. Nat Commun. 2024. PMID: 38278864 Free PMC article. - Dengue virus downregulates TNFR1- and TLR3-stimulated NF-κB activation by targeting RIPK1.
Udawatte DJ, Lang DM, Currier JR, Medin CL, Rothman AL. Udawatte DJ, et al. Front Cell Infect Microbiol. 2022 Oct 14;12:926036. doi: 10.3389/fcimb.2022.926036. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36310878 Free PMC article. - Going up in flames: necrotic cell injury and inflammatory diseases.
Challa S, Chan FK. Challa S, et al. Cell Mol Life Sci. 2010 Oct;67(19):3241-53. doi: 10.1007/s00018-010-0413-8. Epub 2010 Jun 8. Cell Mol Life Sci. 2010. PMID: 20532807 Free PMC article. Review. - Necroptosis as an alternative form of programmed cell death.
Christofferson DE, Yuan J. Christofferson DE, et al. Curr Opin Cell Biol. 2010 Apr;22(2):263-8. doi: 10.1016/j.ceb.2009.12.003. Epub 2010 Jan 4. Curr Opin Cell Biol. 2010. PMID: 20045303 Free PMC article. Review. - Molecular mechanisms of necroptosis: an ordered cellular explosion.
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Vandenabeele P, et al. Nat Rev Mol Cell Biol. 2010 Oct;11(10):700-14. doi: 10.1038/nrm2970. Epub 2010 Sep 8. Nat Rev Mol Cell Biol. 2010. PMID: 20823910 Review.
References
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. - PubMed
- Meylan E, Tschopp J. The RIP kinases: Crucial integrators of cellular stress. Trends Biochem Sci. 2005;30:151–159. - PubMed
- Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell Death Differ. 2007;14:400–410. - PubMed
- Kelliher MA, et al. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. - PubMed
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous