Structural constraints on autoprocessing of the human nucleoporin Nup98 - PubMed (original) (raw)
Structural constraints on autoprocessing of the human nucleoporin Nup98
Yixin Sun et al. Protein Sci. 2008 Mar.
Abstract
Nucleoporin Nup98, a 98-kDa protein component of the nuclear pore complex, plays an important role in both protein and RNA transport. During its maturation process, Nup98 undergoes post-translational autoproteolysis, which is critical for targeting to the NPC. Here we present high-resolution crystal structures of the C-terminal autoproteolytic domains of Nup98 (2.3 A for the wild type and 1.9 A for the S864A precursor), and propose a detailed autoproteolysis mechanism through an N-O acyl shift. Structural constraints are found at the autocleavage site, and could thus provide a driving force for autocleavage at the scissile peptide bond. Such structural constraints appear to be generated, at least in part, by anchoring a conserved phenylalanine side chain into a highly conserved hydrophobic pocket at the catalytic site. Our high-resolution crystal structures also reveal that three highly conserved residues, Tyr866, Gly867, and Leu868, provide most of the interactions between the autoproteolytic domain and the C-terminal tail. These results suggest that Nup98 may represent a new subtype of protein that utilizes autoprocessing to control biogenesis pathways and intracellular translocation.
Figures
Figure 1.
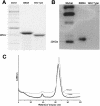
Autoproteolysis activity of the minimized Nup98 domain. (A) Slight migration difference on SDS-PAGE between the wild-type domain (a.a. 712–870 plus six histidines on its C terminus) and the autocleavage-deficient counterpart, the S864A precursor (a.a. 716–870 plus six histidines on its C terminus). The faster mobility of the wild-type fragment suggests an autocleavage has occurred to trim it into a smaller fragment. (B) Western blot results, probed with a His-tag antibody, indicate that the C-terminal His-tagged tail remains attached to the autocleavage-deficient (S864A) fragment but is absent from the wild-type counterpart. (C) FPLC gel filtration curves of the wild-type (gray) and the S864A precursor (black). Significant difference in elution volume is indicative of conformational differences, likely a result of autoproteolysis in the wild-type protein.
Figure 2.
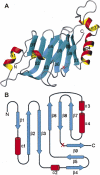
Architecture of the autoproteolytic domain of Nup98. The S864A precursor and wild-type domain have very similar overall structures, containing double layers of β-strands with two α-helices capped on each side. (A) Ribbon representation of the S864A precursor structure, with α-helices and β-strands shown as red/yellow and cyan, respectively. The red “X” marks the autocleavage site between Phe863 and Ser864. Figure produced with MOLMOL (Koradi et al. 1996). (B) Schematic diagram showing the topology of the Nup98 autocatalytic domain. The α-helices are represented by red columns, and the β-strands by cyan arrows.
Figure 3.
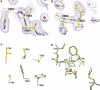
Structural features and comparison at the autocleavage sites of the S864A precursor and the wild-type protein. (A) Electron-density map at the autocleavage site of the S864A precursor. The magenta map is the 2_F_ o–F c map at 1.9 Å resolution and contoured at 1.3σ. Model near the scissile peptide bond, at the carbonyl end of Phe863, is shown by atom type: yellow for carbons, blue for nitrogens, and red for oxygens. The electron density between F863 and A864 is continuous, indicating that S864A is an uncleaved precursor. Also shown are some bound water molecules, but the potential nucleophilic water molecule W97 (discussed in the text) is not shown for clarity. (B) Electron-density map at the autocleavage site of the wild-type domain. Shown is a 2_F_ o–F c map at 2.3 Å resolution of the wild-type structure. The backbone electron density terminates at Phe863, indicating that the C-terminal tail is autocleaved and becomes disordered. Figures produced with PyMOL (
). (C) A conserved hydrophobic pocket near the autocleavage site. The side chain of Phe863 (colored in green) is deeply buried in a hydrophobic pocket surrounded by side chains of I758, V784, V804, V860, and Y866 (yellow for carbons and red for oxygens); these residues are invariant or highly conserved among mammals, insects, and yeast. Additional contribution for the pocket comes from main-chain atoms of V784, H862, S864, and L798 (not shown). Figure was produced with PyMOL. (D) Structural comparison of the Nup98 autocleavage sites before and after autoproteolysis. Residues at the active sites and common to the S864A precursor and the wild-type structures are shown in green and red/yellow/blue, respectively. Structure superimposition was made by Cα atoms of the major β-sheet (including residues 748–760 [strands β2 and β3], 803–807 [strand β6], and 846–858 [strands β7 and β8], see Fig. 2), with an RMSD of 0.2 Å. Figure produced with PyMOL.
Figure 4.
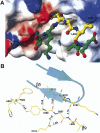
Structural details of the interactions between Nup98-N and the C-terminal tail. (A) Binding of the C-terminal tail to a cleft on the Nup98-N domain. Figure was generated based on the wild-type autocleaved structure. The solvent-accessible surfaces of the Nup98-N domain are colored according to electrostatic potential (blue, positively charged; red, negatively charged; and white, uncharged). Indicated areas are the charged surfaces contributed by the carboxylate terminus of Nup98-N domain (labeled F863-CO2 −) and by residues Lys791, Glu781, and Lys780. The structure of the C-terminal tail is displayed with a ball-and-stick model, using the stable fragment (residues Tyr866–Leu868) from the wild-type structure, whereas the disordered sections (a.a. 864–865 and 869–870) are modeled with the help of the corresponding sections from the precursor structure). The C-terminal tail is colored by atom types: nitrogens in blue, oxygens in red, and carbons in yellow and green for the stable and disordered sections, respectively. The figure was produced by MOLMOL (Koradi et al. 1996). (B) Associations between the C-terminal tail and the Nup98-N domain. The well-ordered section of C-terminal tail (residues Tyr866, Gly867, and Leu868) corresponds to the last strand, β9, in the precursor structure, which forms an antiparallel sheet with strand β5 (residues 780–784) through backbone hydrogen-bond interactions (distances in Å are indicated). In addition, Gly867 also interacts with the side chain of Gln842. Thus these three residues remain stable after autocleavage. Figure was produced with the S864A precursor model with PyMOL.
Figure 5.
Amino acid sequence alignments at the autoproteolytic sites of Nups, GAs, and related proteins. Amino acid sequences are from Saarela et al. (1998), Rosenblum and Blobel (1999), Hsieh et al. (2003), and Tinel et al. (2007). Residues directly involved in catalysis are shown in red. Other conserved residues within subfamilies of homologs are shown in green.
Figure 6.
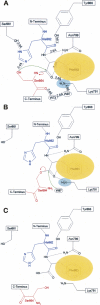
Proposed autoproteolysis mechanism of Nup98. The nucleophilic Ser864 and the general base His862 are shown in red and blue, respectively. The side chain of Phe863 is buried in a conserved hydrophobic pocket (shown as an orange ellipse) to hold the strained scissile peptide bond between Phe863 and Ser864. Nucleophilic attacks are indicated by green curved arrows, and the electron-pair transfers are indicated by red curved arrows. Dashed lines indicate hydrogen bonds. Two bound water molecules near the autocleavage center are displayed in small ellipses (labeled as W73 and W97). The three key elements involved in the autoproteolysis are the nucleophile (the side-chain hydroxyl group of Ser864 for the first nucleophilic attack, and possibly water W97 for the second nucleophilic attack), the oxyanion hole (the positively charged side chain of Lys791, indicated by the cyan ellipse, and possible contribution from the side chain of Asn799), and the general base (side chain of His 862 for the first step). (A) Side chain of Phe863 is anchored into a conserved hydrophobic pocket to maintain the critical structural constraints at the scissile peptide bond and also positions the side chain of His862 to act as the general base and to deprotonate the hydroxyl group of Ser 864 for the first nucleophilic attack. Such a nucleophilic attack at the carbonyl carbon of Phe863 results in a negatively charged tetrahedral transition state, that is stabilized by the oxyanion hole formed by the side chain of Lys791 and possibly the side chain of Asn799. Subsequent collapse of this tetrahedral intermediate results in an N–O acyl arrangement, forming an ester intermediate. (B) The ester intermediate is further hydrolyzed to break the linkage. A bound water molecule (W97) is the likely candidate to make the second nucleophilic attack at the same carbonyl carbon of Phe863, forming the second tetrahedral transition state that is also stabilized by the same oxyanion hole as the first step. Collapse of this second tetrahedral transition state breaks the ester bond and results in the autoproteolyzed Nup98 protein. (C) The autoproteolyzed (mature) form of Nup98, in which the peptide bond between Phe863 and Ser864 is broken, caused conformational changes around the autocatalytic center (see text for details).
Similar articles
- The three-dimensional structure of the autoproteolytic, nuclear pore-targeting domain of the human nucleoporin Nup98.
Hodel AE, Hodel MR, Griffis ER, Hennig KA, Ratner GA, Xu S, Powers MA. Hodel AE, et al. Mol Cell. 2002 Aug;10(2):347-58. doi: 10.1016/s1097-2765(02)00589-0. Mol Cell. 2002. PMID: 12191480 - Vesiculoviral matrix (M) protein occupies nucleic acid binding site at nucleoporin pair (Rae1 • Nup98).
Quan B, Seo HS, Blobel G, Ren Y. Quan B, et al. Proc Natl Acad Sci U S A. 2014 Jun 24;111(25):9127-32. doi: 10.1073/pnas.1409076111. Epub 2014 Jun 9. Proc Natl Acad Sci U S A. 2014. PMID: 24927547 Free PMC article. - Structural and functional analysis of the interaction between the nucleoporin Nup98 and the mRNA export factor Rae1.
Ren Y, Seo HS, Blobel G, Hoelz A. Ren Y, et al. Proc Natl Acad Sci U S A. 2010 Jun 8;107(23):10406-11. doi: 10.1073/pnas.1005389107. Epub 2010 May 24. Proc Natl Acad Sci U S A. 2010. PMID: 20498086 Free PMC article. - Nucleoporin Nup98: a gatekeeper in the eukaryotic kingdoms.
Iwamoto M, Asakawa H, Hiraoka Y, Haraguchi T. Iwamoto M, et al. Genes Cells. 2010 Jun;15(7):661-9. doi: 10.1111/j.1365-2443.2010.01415.x. Epub 2010 Jun 7. Genes Cells. 2010. PMID: 20545767 Review. - NUP98 fusion in human leukemia: dysregulation of the nuclear pore and homeodomain proteins.
Nakamura T. Nakamura T. Int J Hematol. 2005 Jul;82(1):21-7. doi: 10.1532/IJH97.04160. Int J Hematol. 2005. PMID: 16105755 Review.
Cited by
- Advances in the understanding of nuclear pore complexes in human diseases.
Li Y, Zhu J, Zhai F, Kong L, Li H, Jin X. Li Y, et al. J Cancer Res Clin Oncol. 2024 Jul 30;150(7):374. doi: 10.1007/s00432-024-05881-5. J Cancer Res Clin Oncol. 2024. PMID: 39080077 Free PMC article. Review. - An ancient autoproteolytic domain found in GAIN, ZU5 and Nucleoporin98.
Liao Y, Pei J, Cheng H, Grishin NV. Liao Y, et al. J Mol Biol. 2014 Dec 12;426(24):3935-3945. doi: 10.1016/j.jmb.2014.10.011. Epub 2014 Oct 20. J Mol Biol. 2014. PMID: 25451782 Free PMC article. - The yeast nuclear pore complex and transport through it.
Aitchison JD, Rout MP. Aitchison JD, et al. Genetics. 2012 Mar;190(3):855-83. doi: 10.1534/genetics.111.127803. Genetics. 2012. PMID: 22419078 Free PMC article. - Evolutionary divergence of the nuclear pore complex from fungi to metazoans.
Chopra K, Bawaria S, Chauhan R. Chopra K, et al. Protein Sci. 2019 Mar;28(3):571-586. doi: 10.1002/pro.3558. Epub 2018 Dec 24. Protein Sci. 2019. PMID: 30488506 Free PMC article. - CARD8 and NLRP1 undergo autoproteolytic processing through a ZU5-like domain.
D'Osualdo A, Weichenberger CX, Wagner RN, Godzik A, Wooley J, Reed JC. D'Osualdo A, et al. PLoS One. 2011;6(11):e27396. doi: 10.1371/journal.pone.0027396. Epub 2011 Nov 8. PLoS One. 2011. PMID: 22087307 Free PMC article.
References
- Benjannet, S., Rhainds, D., Essalmani, R., Mayne, J., Wickham, L., Jin, W., Asselin, M.C., Hamelin, J., Varret, M., Allard, D., et al. NARC-1/PCSK9 and its natural mutants: Zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 2004;279:48865–48875. - PubMed
- Blevins, M.B., Smith, A.M., Phillips, E.M., Powers, M.A. Complex formation among the RNA export proteins Nup98, Rae1/Gle2, and TAP. J. Biol. Chem. 2003;278:20979–20988. - PubMed
- Brunger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 1998;54:905–921. - PubMed
- Ditzel, L., Huber, R., Mann, K., Heinemeyer, W., Wolf, D.H., Groll, M. Conformational constraints for protein self-cleavage in the proteasome. J. Mol. Biol. 1998;279:1187–1191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases