CD36 deficiency leads to choroidal involution via COX2 down-regulation in rodents - PubMed (original) (raw)
Comparative Study
doi: 10.1371/journal.pmed.0050039.
William Raoul, Sophie Lavalette, Nicole Keller, Xavier Guillonneau, Barbara Baragatti, Laurent Jonet, Jean-Claude Jeanny, Francine Behar-Cohen, Flavio Coceani, Daniel Scherman, Pierre Lachapelle, Huy Ong, Sylvain Chemtob, Florian Sennlaub
Affiliations
- PMID: 18288886
- PMCID: PMC2245984
- DOI: 10.1371/journal.pmed.0050039
Comparative Study
CD36 deficiency leads to choroidal involution via COX2 down-regulation in rodents
Marianne Houssier et al. PLoS Med. 2008 Feb.
Abstract
Background: In the Western world, a major cause of blindness is age-related macular degeneration (AMD). Recent research in angiogenesis has furthered the understanding of choroidal neovascularization, which occurs in the "wet" form of AMD. In contrast, very little is known about the mechanisms of the predominant, "dry" form of AMD, which is characterized by retinal atrophy and choroidal involution. The aim of this study is to elucidate the possible implication of the scavenger receptor CD36 in retinal degeneration and choroidal involution, the cardinal features of the dry form of AMD.
Methods and findings: We here show that deficiency of CD36, which participates in outer segment (OS) phagocytosis by the retinal pigment epithelium (RPE) in vitro, leads to significant progressive age-related photoreceptor degeneration evaluated histologically at different ages in two rodent models of CD36 invalidation in vivo (Spontaneous hypertensive rats (SHR) and CD36-/- mice). Furthermore, these animals developed significant age related choroidal involution reflected in a 100%-300% increase in the avascular area of the choriocapillaries measured on vascular corrosion casts of aged animals. We also show that proangiogenic COX2 expression in RPE is stimulated by CD36 activating antibody and that CD36-deficient RPE cells from SHR rats fail to induce COX2 and subsequent vascular endothelial growth factor (VEGF) expression upon OS or antibody stimulation in vitro. CD36-/- mice express reduced levels of COX2 and VEGF in vivo, and COX2-/- mice develop progressive choroidal degeneration similar to what is seen in CD36 deficiency.
Conclusions: CD36 deficiency leads to choroidal involution via COX2 down-regulation in the RPE. These results show a novel molecular mechanism of choroidal degeneration, a key feature of dry AMD. These findings unveil a pathogenic process, to our knowledge previously undescribed, with important implications for the development of new therapies.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
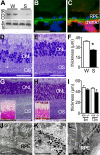
Figure 1. Retinal Degeneration in CD36-Deficient Animals
(A) CD36 Western blot analysis of RPE/choroids complexes from Wistar rats (W) and SHRs S) (n = 4 eyes per group). (B and C) CD36 expression (green fluorescence) (B) and double-labeling with vascular marker BSA-1 (CD36 [green], BSA-1 [red], DAPI [blue]) (C) in CD36+/+ mice (representative picture of three independent experiments). (D and E) Hemalun stained semi thin sections of 10-mo-old CD36-deficient SHRs (D) and control Wistar (W) rats (E). (F) Outer nuclear layer measurements of 10-mo-old Wistar rats (W; n = 6) SHRs (S; n = 8) (*p < 0.0001). (G and H) Hemalun-stained semi-thin sections (and periodic acid Schiff-stained paraffin sections [inset]) of 1-y-old CD36−/− mice (G) and age-matched WT mice (H). (I) ONL thickness measurements in eyes of CD36−/− (black bars; n = 10) and CD36+/+ (white columns; n = 6) mice at different ages (*p = 0.0095 significant difference at 12 mo). (J–L) Transmission electron microscopy of the RPE and outer segments in SHRs (J), and CD36−/− mice (K) and a CD36-expressing congener strain (CD36+/+ mice) (L). Results are representative of at least three independent experiments. Scale bar: B, C, D, E, G, and H = 50 μm; J–L = 5μm.
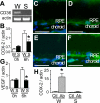
Figure 2. Choroidal Degeneration in CD36-Deficient Animals
(A and B) Micrographs of the retinal aspect of choriocapillaries in a frontal view of corrosion casts by scanning electron microscopy. Choroidal vessels (darker grey) can be seen through the intercapillary spaces of the choriocapillaries in 4-mo-old SHRs (A) but not in age-matched Wistar rats (B). (C) Quantification of intercapillary space expressed as avascular area in 4-mo-old Wistar rats (W) and SHRs (S) (n = 5 eyes/group; *p = 0.0027). (D and E) Frontal view of the retinal aspect of choriocapillaries of 12-mo-old CD36−/− (D) and CD36+/+ (E) mice show defects in the capillary bed of CD36−/− mice. (F) Quantification of the avascular area over time (ages 4 mo versus 12 mo) of CD36+/+ (n = 6) and CD36−/− (n = 8) mouse eyes; *p = 0.0286 significant difference at 12 mo). (G and H) Perpendicular view of choriocapillaries (indicated between arrows) and large choroidal vessels on cross-sectional cuts of pericentral area of CD36−/− (G) and CD36+/+ control (H) mice. (I and J) Transmission electron microscopy of choriocapillaries of CD36−/− (arrow) (I) and CD36+/+ (J) mice. (K) Quantification of capillary thickness of 12-mo-old CD36−/− (n = 10) and CD36+/+ (n = 8) mouse eyes (*p = 0.0062). Results are representative of at least three independent experiments. +/+, wild-type animals, white columns; −/−, CD36-deficient animals, black columns; m, month; ON, optic nerve. Scale bar: A, B, D, E, G, and H = 100 μm; I and J = 5 μm.
Figure 3. OS-Induced COX2 and VEGF Expression in RPE is CD36 Dependent
(A–G) RT-PCR of cDNA from primary RPE cultures from Wistar rats and SHRs (A). Relative COX2 (B) and VEGF (G) mRNA expression (measured by real time RT-PCR. n = 6 wells per group; (B) *p = 0.0152 significant difference between control and CD36-deficient rats at 6 h; (G) *p = 0.0087 significant difference at 6 h) in RPE cells of Wistar (W) and SHR (S) rats exposed in culture to rod outer segments. COX2 (C and D) and VEGF (E and F) immunoreactivity (green) in 4-mo-old CD36−/− (C and E) and CD36+/+ (D and F) mice; tissues were counterstained with DAPI (nuclear stain). (H) Activation of CD36 with stimulating antibody evoked COX2 expression on RPE cell cultures from Wistar rats (W) and SHRs (measured by real time RT-PCR; n = 6 wells per group; *p = 0.0012 COX2 expression significantly different between control [Ctl] and antibody-treated [Ab] Wistar RPE culture). Photographs of immunohistochemical signal were taken with identical parameters in CD36−/− and CD36+/+ mice. Results are representative of at least three independent experiments. Ab, CD36 antibody FA6–152; CTL, control; RPE, retinal pigment epithelium. Scale bar: 50 μm
Figure 4. Choroidal Involution in COX2−/− Mice
(A and B) Micrographs of the retinal aspect of choriocapillaries in a frontal view of corrosion casts by scanning electron microscopy of 12-mo-old COX2−/− (A) and COX2+/+ (B) mice. (C–E) Quantification of the avascular area (n = 6 COX2+/+ and n = 8 COX2−/− eyes; *p = 0.007 COX2−/− significantly different from COX2+/+ at 12 mo) (C). Cross-sectional cuts of pericentral choroidal corrosion casts of COX2−/− (D) and COX2+/+ (E) mice. (F) Quantification of capillary thickness of 12-mo-old COX2+/+ and COX2−/− mice (n = 6 COX2+/+ and n = 8 COX2−/− eyes; *p = 0.0007). (G–I) Relative VEGF mRNA expression (by real time RT-PCR; n = 8 wells per group; *p = 0.0029 rod outer segments with DUP697 [R+D] significantly different from rod outer segments alone [R]) in primary RPE culture of Wistar rats (Ctl, white column), exposed to rod outer segments in absence (R, black column) or presence of the COX2 inhibitor DUP697 (10−6 M) (R+D, hatched column) (G). VEGF expression in 4-mo-old COX2−/− (H) and COX2+/+ (I) mice. Photomicrographs of immunohistochemical signal were taken with identical parameters. Results are representative of at least three independent experiments. m, months; RPE, retinal pigment epithelium. Scale bar: A, B, D, and E = 100 μm; H and I = 100 μm.
Similar articles
- Aryl hydrocarbon receptor knock-out exacerbates choroidal neovascularization via multiple pathogenic pathways.
Choudhary M, Kazmin D, Hu P, Thomas RS, McDonnell DP, Malek G. Choudhary M, et al. J Pathol. 2015 Jan;235(1):101-12. doi: 10.1002/path.4433. Epub 2014 Oct 10. J Pathol. 2015. PMID: 25186463 Free PMC article. - Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model.
Kim SY, Kambhampati SP, Bhutto IA, McLeod DS, Lutty GA, Kannan RM. Kim SY, et al. Exp Eye Res. 2021 Feb;203:108391. doi: 10.1016/j.exer.2020.108391. Epub 2020 Dec 8. Exp Eye Res. 2021. PMID: 33307075 - Autoimmune-Mediated Retinopathy in CXCR5-Deficient Mice as the Result of Age-Related Macular Degeneration Associated Proteins Accumulation.
Lennikov A, Saddala MS, Mukwaya A, Tang S, Huang H. Lennikov A, et al. Front Immunol. 2019 Aug 14;10:1903. doi: 10.3389/fimmu.2019.01903. eCollection 2019. Front Immunol. 2019. PMID: 31474986 Free PMC article. - [Molecular mechanism of choroidal neovascularization in age-related macular degeneration].
Yoshida T. Yoshida T. Nippon Ganka Gakkai Zasshi. 2007 Nov;111(11):881-91. Nippon Ganka Gakkai Zasshi. 2007. PMID: 18051818 Review. Japanese.
Cited by
- High glucose promotes the migration of retinal pigment epithelial cells through increased oxidative stress and PEDF expression.
Farnoodian M, Halbach C, Slinger C, Pattnaik BR, Sorenson CM, Sheibani N. Farnoodian M, et al. Am J Physiol Cell Physiol. 2016 Sep 1;311(3):C418-36. doi: 10.1152/ajpcell.00001.2016. Epub 2016 Jul 20. Am J Physiol Cell Physiol. 2016. PMID: 27440660 Free PMC article. - The association of CD36 variants with polypoidal choroidal vasculopathy compared to typical neovascular age-related macular degeneration.
Bessho H, Honda S, Kondo N, Kusuhara S, Tsukahara Y, Negi A. Bessho H, et al. Mol Vis. 2012;18:121-7. Epub 2012 Jan 18. Mol Vis. 2012. PMID: 22275803 Free PMC article. - The ins and outs of cholesterol in the vertebrate retina.
Fliesler SJ, Bretillon L. Fliesler SJ, et al. J Lipid Res. 2010 Dec;51(12):3399-413. doi: 10.1194/jlr.R010538. Epub 2010 Sep 22. J Lipid Res. 2010. PMID: 20861164 Free PMC article. Review. - Mechanisms that minimize retinal impact of apolipoprotein E absence.
Saadane A, Petrov A, Mast N, El-Darzi N, Dao T, Alnemri A, Song Y, Dunaief JL, Pikuleva IA. Saadane A, et al. J Lipid Res. 2018 Dec;59(12):2368-2382. doi: 10.1194/jlr.M090043. Epub 2018 Oct 17. J Lipid Res. 2018. PMID: 30333155 Free PMC article. - Dietary effects on the retina of hamsters.
El-Darzi N, Mast N, Li Y, Pikuleva IA. El-Darzi N, et al. FASEB J. 2025 Mar 31;39(6):e70451. doi: 10.1096/fj.202403390R. FASEB J. 2025. PMID: 40099968 Free PMC article.
References
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. - PubMed
- Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–1535. - PubMed
- Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials