Selective blockade of microRNA processing by Lin28 - PubMed (original) (raw)
Selective blockade of microRNA processing by Lin28
Srinivas R Viswanathan et al. Science. 2008.
Abstract
MicroRNAs (miRNAs) play critical roles in development, and dysregulation of miRNA expression has been observed in human malignancies. Recent evidence suggests that the processing of several primary miRNA transcripts (pri-miRNAs) is blocked posttranscriptionally in embryonic stem cells, embryonal carcinoma cells, and primary tumors. Here we show that Lin28, a developmentally regulated RNA binding protein, selectively blocks the processing of pri-let-7 miRNAs in embryonic cells. Using in vitro and in vivo studies, we found that Lin28 is necessary and sufficient for blocking Microprocessor-mediated cleavage of pri-let-7 miRNAs. Our results identify Lin28 as a negative regulator of miRNA biogenesis and suggest that Lin28 may play a central role in blocking miRNA-mediated differentiation in stem cells and in certain cancers.
Figures
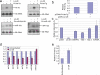
Figure 1. Post-transcriptional control of pri-let-7g processing
a, RT-PCR for pri-let-7g transcript (as described in ref. (7)) during ES differentiation to embryoid bodies. Actin serves as control**. b**, Northern blot showing post-transcriptional induction of mature let-7g during embryoid body formation 5S rRNA serves as loading control. c, in vitro pri-miRNA processing reaction using radiolabeled pri-let-7g as substrate. Pri-miRNA was pre-incubated with various amounts of P19 cell extract or mouse embryonic fibroblast (MEF) extract prior to processing reaction with Flag-Drosha immunoprecipitate, as described in Methods. The ratio of pre-miRNA to pri-miRNA was quantitated by densitometry and values were normalized to the Microprocessor only lane. d, qPCR analysis of gene expression during embryoid body formation of a feeder-free mouse ES line (J1 ES) . Top Panel: Pri-let-7g and mature let-7g; Middle Panel: Lin-28; Bottom Panel: pluripotency factors Oct-4 and Nanog.
Figure 2. Lin-28 Inhibits pri-miRNA processing in vitro
a, α–Flag-Western to confirm expression of Flag-tagged proteins for use in in vitro assays. b, in vitro pri-miRNA processing reaction on pri-let-7g (left panel) and pri-let-7a (right panel) substrates in the presence of either Mock, Flag-Lin-28, Flag-hnRNPA1, or Flag-Msi-2 immunoprecipitate. Quantitation was normalized to the Microprocessor-only lane. c, in vitro pri-miRNA processing reaction on pri-let-7g (left panel) and pri-miR-15a/16-1 (right panel) substrates in the presence of either mock or Flag-Lin-28 immunoprecipitate and competitor tRNA. Quantitation was normalized to the Mock-IP lane. d, in vitro pri-miRNA processing reaction on pri-let-7g (left panel) and pri-let-7a (right panel) substrates in the presence of rHis-Lin-28. Quantitation was normalized to the Microprocessor-only lane.
Figure 3. Ectopic expression of Lin-28 selectively inhibits pri-miRNA processing in vivo
a, In each panel, 293T cells were either untransfected (lane 1), co-transfected with the indicated pri-miRNA and 0.5 μg pCMV-Flag empty vector (lane 2), or co-transfected with the indicated pri-miRNA and 0.5 μg Flag-Lin-28 cDNA (lane 3). Total RNA was collected 40 h post-transfection and Northern blotted for the indicated miRNA. b, qPCR analysis of pri-let-7g levels for sample in a) (top right panel). c, Mature let-7g levels upon co-transfection of 293T cells with pri-let-7g and either pCMV-Flag, Flag-Lin-28, Flag-hnRNPA1, Flag-hnRNPL, Flag-YBX-1, or Flag-Msi-2 cDNAs, as measured by quantitative PCR. First, the amount of mature let-7g in each sample was calculated relative to untransfected control cells, then Flag-protein co-transfected samples were normalized to the corresponding pCMV-Flag co-transfected samples. d, qPCR showing changes in levels of endogenous mature miRNAs upon transfection of Flag-Lin-28 in 293T cells. e, qPCR showing accumulation of endogenous pri-let-7g upon transfection of Flag-Lin-28 in 293T cells. For c-e, values are given as average +/- S.E.M. from two or more independent transfections.
Figure 4. Knockdown of Lin-28 relieves the miRNA-processing block
P19 cells were transfected with control hairpin (GFPi), pLKO.1-shRNA hairpins targeting Lin-28, control siRNA (scrambled sequence), or Lin-28 siRNA. Total RNA was collected 60-hrs post-transfection for analysis. a, quantitative PCR analysis of Lin-28 expression, normalized to Lin-28 expression with control hairpin or control siRNA, for samples in b. b, Northern blot for mature let-7g. c, confirmation of Lin-28 knockdown using Lin28-SI2 on samples analyzed in d. Error bars represent S.E.M. with N=3. d, Changes in mature miRNA levels upon knockdown of Lin-28 as analyzed by quantitative PCR. Error bars represent S.E.M. with N=3. e, levels of pri-let-7g upon knockdown of Lin-28 in P19 cells. Error bars represent S.E.M. with N=3. f, levels of the pluripotency markers Oct-4 and Nanog in P19 cells transfected with either control siRNA or Lin28-SI2. Error bars represent S.E.M. with N=3.
Comment in
- Development. Deconstructing pluripotency.
Bang AG, Carpenter MK. Bang AG, et al. Science. 2008 Apr 4;320(5872):58-9. doi: 10.1126/science.1157042. Science. 2008. PMID: 18388280 No abstract available.
Similar articles
- Musashi1 cooperates in abnormal cell lineage protein 28 (Lin28)-mediated let-7 family microRNA biogenesis in early neural differentiation.
Kawahara H, Okada Y, Imai T, Iwanami A, Mischel PS, Okano H. Kawahara H, et al. J Biol Chem. 2011 May 6;286(18):16121-30. doi: 10.1074/jbc.M110.199166. Epub 2011 Mar 4. J Biol Chem. 2011. PMID: 21378162 Free PMC article. - Identification of microRNAs regulating reprogramming factor LIN28 in embryonic stem cells and cancer cells.
Zhong X, Li N, Liang S, Huang Q, Coukos G, Zhang L. Zhong X, et al. J Biol Chem. 2010 Dec 31;285(53):41961-71. doi: 10.1074/jbc.M110.169607. Epub 2010 Oct 14. J Biol Chem. 2010. PMID: 20947512 Free PMC article. - Mature Let-7 miRNAs fine tune expression of LIN28B in pluripotent human embryonic stem cells.
Rahkonen N, Stubb A, Malonzo M, Edelman S, Emani MR, Närvä E, Lähdesmäki H, Ruohola-Baker H, Lahesmaa R, Lund R. Rahkonen N, et al. Stem Cell Res. 2016 Nov;17(3):498-503. doi: 10.1016/j.scr.2016.09.025. Epub 2016 Sep 24. Stem Cell Res. 2016. PMID: 27776272 - Embryonic stem cell microRNAs: defining factors in induced pluripotent (iPS) and cancer (CSC) stem cells?
Gunaratne PH. Gunaratne PH. Curr Stem Cell Res Ther. 2009 Sep;4(3):168-77. doi: 10.2174/157488809789057400. Curr Stem Cell Res Ther. 2009. PMID: 19492978 Review. - Concise Review: LIN28/let-7 Signaling, a Critical Double-Negative Feedback Loop During Pluripotency, Reprogramming, and Tumorigenicity.
Farzaneh M, Attari F, Khoshnam SE. Farzaneh M, et al. Cell Reprogram. 2017 Oct;19(5):289-293. doi: 10.1089/cell.2017.0015. Epub 2017 Aug 28. Cell Reprogram. 2017. PMID: 28846452 Review.
Cited by
- MicroRNAs and liver disease.
Otsuka M, Kishikawa T, Yoshikawa T, Yamagami M, Ohno M, Takata A, Shibata C, Ishibashi R, Koike K. Otsuka M, et al. J Hum Genet. 2017 Jan;62(1):75-80. doi: 10.1038/jhg.2016.53. Epub 2016 May 26. J Hum Genet. 2017. PMID: 27225852 Review. - Bridges between Cell Cycle Regulation and Self-Renewal Maintenance.
Viatour P. Viatour P. Genes Cancer. 2012 Nov;3(11-12):670-7. doi: 10.1177/1947601913481355. Genes Cancer. 2012. PMID: 23634255 Free PMC article. - MicroRNA let-7c is downregulated in prostate cancer and suppresses prostate cancer growth.
Nadiminty N, Tummala R, Lou W, Zhu Y, Shi XB, Zou JX, Chen H, Zhang J, Chen X, Luo J, deVere White RW, Kung HJ, Evans CP, Gao AC. Nadiminty N, et al. PLoS One. 2012;7(3):e32832. doi: 10.1371/journal.pone.0032832. Epub 2012 Mar 30. PLoS One. 2012. PMID: 22479342 Free PMC article. - Inactivation of ribosomal protein L22 promotes transformation by induction of the stemness factor, Lin28B.
Rao S, Lee SY, Gutierrez A, Perrigoue J, Thapa RJ, Tu Z, Jeffers JR, Rhodes M, Anderson S, Oravecz T, Hunger SP, Timakhov RA, Zhang R, Balachandran S, Zambetti GP, Testa JR, Look AT, Wiest DL. Rao S, et al. Blood. 2012 Nov 1;120(18):3764-73. doi: 10.1182/blood-2012-03-415349. Epub 2012 Sep 13. Blood. 2012. PMID: 22976955 Free PMC article. - Control by a hair's breadth: the role of microRNAs in the skin.
Ning MS, Andl T. Ning MS, et al. Cell Mol Life Sci. 2013 Apr;70(7):1149-69. doi: 10.1007/s00018-012-1117-z. Epub 2012 Sep 15. Cell Mol Life Sci. 2013. PMID: 22983383 Free PMC article. Review.
References
- Denli AM, Tops BBJ, Plasterk RHA, Ketting RF, Hannon GJ. Nature. 2004;432:231–235. - PubMed
- Gregory RI, et al. Nature. 2004;432:235–240. - PubMed
- Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Cell. 2005;123:631–640. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials