Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging - PubMed (original) (raw)
Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging
Christopher M Holland et al. Stroke. 2008 Apr.
Abstract
Background and purpose: White-matter hyperintensities (WMHs) detected by magnetic resonance imaging are thought to represent the effects of cerebral small-vessel disease and neurodegenerative changes. We sought to determine whether the spatial distribution of WMHs discriminates between different disease groups and healthy aging individuals and whether these distributions are related to local cerebral perfusion patterns.
Methods: We examined the pattern of WMHs by T2/fluid-attenuated inversion recovery-weighted magnetic resonance imaging in 3 groups of subjects: cerebral amyloid angiopathy (n=32), Alzheimer disease or mild cognitive impairment (n=41), and healthy aging (n=29). WMH frequency maps were calculated for each group, and spatial distributions were compared by voxel-wise logistic regression. WMHs were also analyzed as a function of normal cerebral perfusion patterns by overlaying a single photon emission computed tomography atlas.
Results: Although WMH volume was greater in cerebral amyloid angiopathy and Alzheimer disease/mild cognitive impairment than in healthy aging, there was no consistent difference in the spatial distributions when controlling for total WMH volume. Hyperintensities were most frequent in the deep periventricular WM in all 3 groups. A strong inverse correlation between hyperintensity frequency and normal perfusion was demonstrated in all groups, demonstrating that WMHs were most common in regions of relatively lower normal cerebral perfusion.
Conclusions: WMHs show a common distribution pattern and predilection for cerebral WM regions with lower atlas-derived perfusion, regardless of the underlying diagnosis. These data suggest that across diverse disease processes, WM injury may occur in a pattern that reflects underlying tissue properties, such as relative perfusion.
Figures
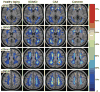
Figure 1
Atlas of normal cerebral WM perfusion. SPECT perfusion atlas generated from 47 healthy adult volunteers was masked by a coregistered WM probability atlas. (A conservative threshold of 0.75 was used for illustrative purposes only.) Perfusion values are indicated by the color bar, in which red indicates higher relative perfusion (scale, relative perfusion units).
Figure 2
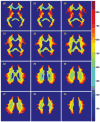
Spatial distribution of WMHs. The WMH frequency distributions for the HA, AD/MCI, and CAA cohorts were overlaid onto the International Consortium of Brain Mapping template to which all individual subject MRI images were registered. WMH frequency corresponds to the proportion of subjects in each cohort having WMHs in that image voxel. The fourth column represents the regions of WMHs common to all subjects, with the relative frequency across the entire study population.
Figure 3
Statistical comparison of WMH distributions. WMH distributions were compared between the 3 groups by voxel-wise logistic regression, with and without controlling for total WMH volume in each subject. Voxels in which the prevalence of WMHs differed significantly (P<0.05) are presented according to the group with the higher WMH prevalence: HA higher (green), AD/MCI higher (red), and CAA higher (blue). Three representative slices are shown.
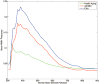
Figure 4
WMH frequency histogram with respect to normal atlas-derived perfusion for the HA (green), AD/MCI (red), and CAA (blue) cohorts. The increased prevalence of hyperintensity in regions of relatively lower normal perfusion is evident (Pearson’s _r_=−0.54, P<0.001 across all subjects).
Similar articles
- Association of Data-Driven White Matter Hyperintensity Spatial Signatures With Distinct Cerebral Small Vessel Disease Etiologies.
Phuah CL, Chen Y, Strain JF, Yechoor N, Laurido-Soto OJ, Ances BM, Lee JM; Alzheimer's Disease Neuroimaging Initiative. Phuah CL, et al. Neurology. 2022 Dec 5;99(23):e2535-e2547. doi: 10.1212/WNL.0000000000201186. Neurology. 2022. PMID: 36123127 Free PMC article. - White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease?
Provenzano FA, Muraskin J, Tosto G, Narkhede A, Wasserman BT, Griffith EY, Guzman VA, Meier IB, Zimmerman ME, Brickman AM; Alzheimer's Disease Neuroimaging Initiative. Provenzano FA, et al. JAMA Neurol. 2013 Apr;70(4):455-61. doi: 10.1001/jamaneurol.2013.1321. JAMA Neurol. 2013. PMID: 23420027 Free PMC article. - Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD.
Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Yoshita M, et al. Neurology. 2006 Dec 26;67(12):2192-8. doi: 10.1212/01.wnl.0000249119.95747.1f. Neurology. 2006. PMID: 17190943 Free PMC article. - Current concepts of analysis of cerebral white matter hyperintensities on magnetic resonance imaging.
Yoshita M, Fletcher E, DeCarli C. Yoshita M, et al. Top Magn Reson Imaging. 2005 Dec;16(6):399-407. doi: 10.1097/01.rmr.0000245456.98029.a8. Top Magn Reson Imaging. 2005. PMID: 17088690 Free PMC article. Review. - Spatial patterns of white matter hyperintensities: a systematic review.
Botz J, Lohner V, Schirmer MD. Botz J, et al. Front Aging Neurosci. 2023 May 11;15:1165324. doi: 10.3389/fnagi.2023.1165324. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37251801 Free PMC article. Review.
Cited by
- An investigation of cerebrovascular lesions in dementia with Lewy bodies compared to Alzheimer's disease.
Sarro L, Tosakulwong N, Schwarz CG, Graff-Radford J, Przybelski SA, Lesnick TG, Zuk SM, Reid RI, Raman MR, Boeve BF, Ferman TJ, Knopman DS, Comi G, Filippi M, Murray ME, Parisi JE, Dickson DW, Petersen RC, Jack CR Jr, Kantarci K. Sarro L, et al. Alzheimers Dement. 2017 Mar;13(3):257-266. doi: 10.1016/j.jalz.2016.07.003. Epub 2016 Aug 10. Alzheimers Dement. 2017. PMID: 27521790 Free PMC article. - Leukoaraiosis and stroke.
Smith EE. Smith EE. Stroke. 2010 Oct;41(10 Suppl):S139-43. doi: 10.1161/STROKEAHA.110.596056. Stroke. 2010. PMID: 20876490 Free PMC article. Review. - Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy.
Biffi A, Halpin A, Towfighi A, Gilson A, Busl K, Rost N, Smith EE, Greenberg MS, Rosand J, Viswanathan A. Biffi A, et al. Neurology. 2010 Aug 24;75(8):693-8. doi: 10.1212/WNL.0b013e3181eee40f. Neurology. 2010. PMID: 20733144 Free PMC article. - Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations.
Yates PA, Villemagne VL, Ellis KA, Desmond PM, Masters CL, Rowe CC. Yates PA, et al. Front Neurol. 2014 Jan 6;4:205. doi: 10.3389/fneur.2013.00205. eCollection 2014 Jan 6. Front Neurol. 2014. PMID: 24432010 Free PMC article. Review. - Ethnoracial differences in brain structure change and cognitive change.
Gavett BE, Fletcher E, Harvey D, Farias ST, Olichney J, Beckett L, DeCarli C, Mungas D. Gavett BE, et al. Neuropsychology. 2018 Jul;32(5):529-540. doi: 10.1037/neu0000452. Epub 2018 Apr 12. Neuropsychology. 2018. PMID: 29648842 Free PMC article.
References
- Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50:972–978. - PubMed
- Meyer JS, Kawamura J, Terayama Y. White matter lesions in the elderly. J Neurol Sci. 1992;110:1–7. - PubMed
- Schmidt R, Schmidt H, Kapeller P, Enzinger C, Ropele S, Saurugg R, Fazekas F. The natural course of MRI white matter hyperintensities. J Neurol Sci. 2002;203(204):253–257. - PubMed
- Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, van Harskamp F, Tanghe HL, de Jong PT, van Gijn J, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44:1246–1252. - PubMed
- Burton EJ, McKeith IG, Burn DJ, Firbank MJ, O’Brien JT. Progression of white matter hyperintensities in Alzheimer disease, dementia with Lewy bodies, and Parkinson disease dementia: a comparison with normal aging. Am J Geriatr Psychiatry. 2006;14:842–849. - PubMed
Publication types
MeSH terms
Grants and funding
- P01 AG004953-200006/AG/NIA NIH HHS/United States
- K24 NS056207/NS/NINDS NIH HHS/United States
- P41 RR13218-01/RR/NCRR NIH HHS/United States
- F30 NS049808-02/NS/NINDS NIH HHS/United States
- R01 AG026484-01/AG/NIA NIH HHS/United States
- P01 AG004953-220006/AG/NIA NIH HHS/United States
- P41 RR013218/RR/NCRR NIH HHS/United States
- R01 AG026484-05/AG/NIA NIH HHS/United States
- P41 RR013218-010009/RR/NCRR NIH HHS/United States
- P01 AG04953/AG/NIA NIH HHS/United States
- P01 AG004953-230006/AG/NIA NIH HHS/United States
- K24 NS056207-03/NS/NINDS NIH HHS/United States
- F30 NS049808-03/NS/NINDS NIH HHS/United States
- R01 AG026484/AG/NIA NIH HHS/United States
- K24 NS056207-04/NS/NINDS NIH HHS/United States
- R01 AG026484-04/AG/NIA NIH HHS/United States
- K24 NS056207-01/NS/NINDS NIH HHS/United States
- P01 AG004953-210006/AG/NIA NIH HHS/United States
- F30 NS049808/NS/NINDS NIH HHS/United States
- R01 AG026484-02/AG/NIA NIH HHS/United States
- P01 AG004953/AG/NIA NIH HHS/United States
- P01 AG004953-190006/AG/NIA NIH HHS/United States
- K24 NS056207-02/NS/NINDS NIH HHS/United States
- F30 NS049808-01A2/NS/NINDS NIH HHS/United States
- R01 AG026484-03/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical