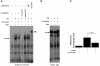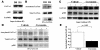The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats - PubMed (original) (raw)
The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats
Tatsuya Morimoto et al. J Clin Invest. 2008 Mar.
Abstract
Hemodynamic overload in the heart can trigger maladaptive hypertrophy of cardiomyocytes. A key signaling event in this process is nuclear acetylation by histone deacetylases and p300, an intrinsic histone acetyltransferase (HAT). It has been previously shown that curcumin, a polyphenol responsible for the yellow color of the spice turmeric, possesses HAT inhibitory activity with specificity for the p300/CREB-binding protein. We found that curcumin inhibited the hypertrophy-induced acetylation and DNA-binding abilities of GATA4, a hypertrophy-responsive transcription factor, in rat cardiomyocytes. Curcumin also disrupted the p300/GATA4 complex and repressed agonist- and p300-induced hypertrophic responses in these cells. Both the acetylated form of GATA4 and the relative levels of the p300/GATA4 complex markedly increased in rat hypertensive hearts in vivo. The effects of curcumin were examined in vivo in 2 different heart failure models: hypertensive heart disease in salt-sensitive Dahl rats and surgically induced myocardial infarction in rats. In both models, curcumin prevented deterioration of systolic function and heart failure-induced increases in both myocardial wall thickness and diameter. From these results, we conclude that inhibition of p300 HAT activity by the nontoxic dietary compound curcumin may provide a novel therapeutic strategy for heart failure in humans.
Figures
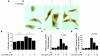
Figure 1. Curcumin inhibits PE-induced hypertrophic responses in cardiomyocytes.
(A and B) Primary cardiomyocytes from neonatal rats were stimulated with saline or 30 μM PE in the presence of curcumin (10 μM in A and 5 or 10 μM in B) or a corresponding amount of its vehicle (DMSO) as a control for 48 hours. (A) These cells were subjected to immunocytochemistry using the primary antibody against cardiac MHC followed by staining with a secondary antibody conjugated with peroxidase (brown signals). Scale bar: 10 μm. (B) Myocardial cell-surface area was measured as described in Methods. Values in each group are mean ± SEM (μm) from 50 cells. (C and D) Cardiomyocytes were transfected with 0.5 μg of pANF-luc (C) or pβ-MHCluc (D) and 0.0025 μg of pRL-SV40. Then, these cells were stimulated with saline or 30 μM PE in the presence of curcumin or a corresponding amount of its vehicle for 48 hours. The relative promoter activities were calculated from the ratio of firefly Luc activity to sea pansy Luc activity. The data shown are mean ± SEM from 3 independent experiments, each carried out in duplicate.
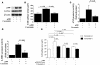
Figure 2. Curcumin suppresses p300-induced hypertrophic responses in cardiomyocytes.
(A and B) Cardiomyocytes were transfected with 0.7 μg of pCMVwtp300 (lanes 2 and 3) or pCMVβ-gal (lane 1) and incubated with curcumin (10 μM, lane 3) or a corresponding amount of its vehicle (lanes 1 and 2) for 48 hours. (A) Protein extracts from these cells were subjected to Western blotting using the anti-p300, anti-GATA4, and anti–β-actin antibodies. (B) Cell-surface area was measured as described in Methods. Values in each group are mean ± SEM (μm) from 50 cells. Cardiomyocytes were cotransfected with 0.5 μg of pANF-luc (C) or pβ-MHCluc (D), 0.0025 μg of pRL-SV40, and 0.25 μg of pCMVwtp300 (lanes 2 and 3) or pCMVβ-gal (lane 1). Then, these cells were incubated with curcumin (10 μM, lane 3) or its vehicle (DMSO, lanes 1 and 2) for 48 hours. The relative promoter activities were calculated from the ratio of firefly Luc to sea pansy Luc and expressed as the mean ± SEM from 3 independent experiments, each carried out in duplicate. (E) Cardiomyocytes were transfected with 0.7 μg of pCMVwtp300 (bars 2 and 3), pCMVHATmutp300 (bars 4 and 5), or pCMVβ-gal (bars 1, 6, and 7) and incubated with TSA (bars 6 and 7), curcumin (10 μM, bars 3, 5, and 7), or a corresponding amount of their vehicles for 48 hours. Cell-surface area was measured as described in Methods. Values in each group are mean ± SEM (μm) from 50 cells.
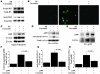
Figure 3. Primary cardiomyocytes from neonatal rats were stimulated with saline (lane 1) or 30 μM of PE (lanes 2 and 3) in the presence of curcumin (10 μM, lane 3) or a corresponding amount of its vehicle (DMSO, lanes 1 and 2) as a control for 48 hours.
(A) Proteins isolated by acid extraction from these cells were subjected to Western blotting for acetylated histone-3/4 or total histone-3/4 as indicated. (B) These cells were subjected to indirect immunofluorescence analysis with antibody against acetylated lysine. Original magnification, ×200. (C) Protein extracts from these cells were subjected to Western blotting with anti-GATA4 antibody, anti-p300 antibody, or anti–β-actin antibody. (D and E) The same extracts (100 μg of protein) were immunoprecipitated with goat anti-GATA4 polyclonal antibody (D) or rabbit anti-p300 polyclonal antibody (E) and subjected to sequential Western blotting with anti–acetylated lysine antibody, anti-p300 antibody, and anti-GATA4 antibody. (F–H) The amounts of GATA4-associated p300/total GATA4 binding (F), acetylated GATA4/total GATA4 (G), and p300-associated GATA4/total p300 binding (H) were quantified by densitometry with the use of Multi Gauge V3.0 (FUJIFILM). The data shown are mean ± SEM from 3 independent experiments.
Figure 4. Curcumin represses PE-induced increase in GATA4-DNA binding.
(A and B) Primary cardiomyocytes from neonatal rats were stimulated with saline (lane 1) or 30 μM of PE (lanes 2 and 3) in the presence of curcumin (10 μM, lane 3) or a corresponding amount of its vehicle (DMSO, lanes 1 and 2) as a control for 48 hours. The nuclear extracts from these cells were probed with a radiolabeled double-stranded oligonucleotide containing the ET-1 GATA site (A) and with one containing the Sp-1 site (B). (C) The amount of GATA4/DNA binding (indicated by a long or short arrow) was quantified by densitometry with the use of NIH Image 1.61. The data shown are mean ± SEM from 3 independent experiments.
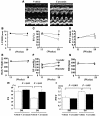
Figure 5. DS rats at the age of 11 weeks were randomly assigned to daily oral treatment with curcumin (50 mg/kg/d) or vehicle (1% gum arabic) for 7 weeks.
Echocardiographic studies were performed at the age of 11 (before treatment) and 18 weeks (after treatment). (A) Representative photographs of M-mode images from vehicle- and curcumin-treated DS rats at the age of 18 weeks. (B–G) Values are mean ± SEM from 6 rats in the curcumin group and 8 rats in the vehicle group. *P < 0.05 vs. vehicle group. (B) LVEDD. (C) FS. (D) PWT, posterior wall thickness. (E) Body weight. (F) BP. (G) HR, heart rate. (H and I) Values are mean ± SEM from 3 rats in each DR group, 8 rats in the vehicle-treated DS group, and 6 rats in the curcumin-treated DS group.
Figure 6. DS and DR rats were treated with curcumin (50 mg/kg/d) or vehicle (1% gum arabic) for 7 weeks.
Then, plasma BNP levels were measured (B), and left ventricles were subjected to real-time PCR analysis for BNP and GAPDH (A) and to histological analysis (C–F). (C) Representative photographs of H&E-stained sections of LV myocardium from vehicle- and curcumin-treated DS rats. Magnification, ×400. Scale bar: 10 μm. (D) Myocardial cell diameters in left ventricles were measured for at least 50 cells in each animal. (E) Representative photographs of Masson trichrome–stained sections of LV myocardium from vehicle- and curcumin-treated DS rats. Original magnification, ×200. Scale bar: 20 μm. (F) Areas of perivascular fibrosis in left ventricles were measured for at least 10 intramyocardial coronary arteries in each animal. Results in A, B, D, and F are expressed as mean ± SEM from 3 rats in each DR group, 8 rats in the vehicle-treated DS group, and 6 rats in the curcumin-treated DS group.
Figure 7. Curcumin inhibits hypertension-induced acetylation of GATA4 as well as p300/GATA4 complex in the LVs of Dahl rats.
(A) Protein extracts from the hearts of DR and DS rats at the age of 18 weeks were subjected to Western blotting with anti-p300 antibody, anti-GATA4 antibody, or anti-GAPDH antibody. (B) These extracts were then immunoprecipitated with goat anti-GATA4 polyclonal antibody and subjected to sequential Western blotting with anti–acetylated lysine antibody, anti-p300 antibody, and anti-GATA4 antibody. (C and D) Protein extracts from the hearts of DS rats treated with curcumin (50 mg/kg/d) or vehicle (1% gum arabic) for 7 weeks were subjected to Western blotting for p300, GATA4, and GAPDH (C) and then immunoprecipitated with anti-GATA4 antibody followed by sequential Western blotting for acetylated lysine, GATA4, and p300 (D). (E) The levels of signals for acetylated GATA4 and those for total GATA4 were quantified. Results are expressed as mean ± SEM from 8 vehicle-treated rats and 6 curcumin-treated rats.
Figure 8. One week after the operation, rats with MI (n = 29) and those that underwent sham operation (n = 14) were treated with curcumin (50 mg/kg/d) or vehicle (1% gum arabic) for 6 weeks.
Echocardiographic studies were performed every 2 weeks. (A) Representative photographs of M-mode images from vehicle- and curcumin-treated MI rats at 7 weeks after the operation. (B–G) Values are mean ± SEM from 14 rats in the curcumin group and 15 rats in the vehicle group. #P < 0.005 vs. vehicle group; *P < 0.0001 vs. vehicle group. (B) LVEDD. (C) FS. (D) Diastolic PWT. (E) Body weight. (F) BP. (G) HR. (H–J) Results are expressed as mean ± SEM from 7 rats in each sham-operated group, 15 rats in the vehicle-treated MI group, and 14 rats in the curcumin-treated MI group. (J) Myocardial cell diameters in left ventricles were measured for at least 50 cells in each animal.
Comment in
- Currying favor for the heart.
Epstein JA. Epstein JA. J Clin Invest. 2008 Mar;118(3):850-2. doi: 10.1172/JCI34650. J Clin Invest. 2008. PMID: 18292806 Free PMC article.
Similar articles
- Curcumin prevents and reverses murine cardiac hypertrophy.
Li HL, Liu C, de Couto G, Ouzounian M, Sun M, Wang AB, Huang Y, He CW, Shi Y, Chen X, Nghiem MP, Liu Y, Chen M, Dawood F, Fukuoka M, Maekawa Y, Zhang L, Leask A, Ghosh AK, Kirshenbaum LA, Liu PP. Li HL, et al. J Clin Invest. 2008 Mar;118(3):879-93. doi: 10.1172/JCI32865. J Clin Invest. 2008. PMID: 18292803 Free PMC article. Retracted. - Novel heart failure therapy targeting transcriptional pathway in cardiomyocytes by a natural compound, curcumin.
Morimoto T, Sunagawa Y, Fujita M, Hasegawa K. Morimoto T, et al. Circ J. 2010 Jun;74(6):1059-66. doi: 10.1253/circj.cj-09-1012. Epub 2010 May 8. Circ J. 2010. PMID: 20467147 Review. - Curcumin, an Inhibitor of p300-HAT Activity, Suppresses the Development of Hypertension-Induced Left Ventricular Hypertrophy with Preserved Ejection Fraction in Dahl Rats.
Sunagawa Y, Funamoto M, Shimizu K, Shimizu S, Sari N, Katanasaka Y, Miyazaki Y, Kakeya H, Hasegawa K, Morimoto T. Sunagawa Y, et al. Nutrients. 2021 Jul 29;13(8):2608. doi: 10.3390/nu13082608. Nutrients. 2021. PMID: 34444769 Free PMC article. - Tyrosine phosphorylation of RACK1 triggers cardiomyocyte hypertrophy by regulating the interaction between p300 and GATA4.
Suzuki H, Katanasaka Y, Sunagawa Y, Miyazaki Y, Funamoto M, Wada H, Hasegawa K, Morimoto T. Suzuki H, et al. Biochim Biophys Acta. 2016 Sep;1862(9):1544-57. doi: 10.1016/j.bbadis.2016.05.006. Epub 2016 May 18. Biochim Biophys Acta. 2016. PMID: 27208796 - Application of curcumin to heart failure therapy by targeting transcriptional pathway in cardiomyocytes.
Katanasaka Y, Sunagawa Y, Hasegawa K, Morimoto T. Katanasaka Y, et al. Biol Pharm Bull. 2013;36(1):13-7. doi: 10.1248/bpb.b212022. Biol Pharm Bull. 2013. PMID: 23302632 Review.
Cited by
- The Anti-Inflammatory Properties of Phytochemicals and Their Effects on Epigenetic Mechanisms Involved in TLR4/NF-κB-Mediated Inflammation.
Saleh HA, Yousef MH, Abdelnaser A. Saleh HA, et al. Front Immunol. 2021 Mar 26;12:606069. doi: 10.3389/fimmu.2021.606069. eCollection 2021. Front Immunol. 2021. PMID: 33868227 Free PMC article. Review. - Alterations of Gut Microbiota by Overnutrition Impact Gluconeogenic Gene Expression and Insulin Signaling.
He L. He L. Int J Mol Sci. 2021 Feb 20;22(4):2121. doi: 10.3390/ijms22042121. Int J Mol Sci. 2021. PMID: 33672754 Free PMC article. Review. - Curcumin Relaxes Precontracted Guinea Pig Gallbladder Strips via Multiple Signaling Pathways.
Kline LW, Karpinski E. Kline LW, et al. Gastroenterology Res. 2015 Oct;8(5):253-259. doi: 10.14740/gr689w. Epub 2015 Oct 21. Gastroenterology Res. 2015. PMID: 27785305 Free PMC article. - Mechanism of histone deacetylases in cardiac hypertrophy and its therapeutic inhibitors.
Han Y, Nie J, Wang DW, Ni L. Han Y, et al. Front Cardiovasc Med. 2022 Jul 26;9:931475. doi: 10.3389/fcvm.2022.931475. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35958418 Free PMC article. Review. - Curcuminoids: Spicing up sympathovagal tone.
Katz PS, Trask AJ, Lucchesi PA. Katz PS, et al. Nutrition. 2009 Jul-Aug;25(7-8):879-80. doi: 10.1016/j.nut.2009.03.007. Nutrition. 2009. PMID: 19539177 Free PMC article. No abstract available.
References
- Ho K.K., Levy D., Kannel W.B., Pinsky J.L. The epidemiology of heart failure: the Framingham study. J. Am. Coll. Cardiol. 1993;22:6–13. - PubMed
- Dominguez L.J., Parrinello G., Amato P., Licata G. Trends of congestive heart failure epidemiology: contrast with clinical trial results. Cardiologia. 1999;44:801–808. - PubMed
- Dorn G.W., II, Robbins J., Sugden P.H. Phenotyping hypertrophy: eschew obfuscation. Circ. Res. 2003;92:1171–1175. - PubMed
- Frey N., Olson E.N. Cardiac hypertrophy: the good, the bad, and the ugly. Annu. Rev. Physiol. 2003;65:45–79. - PubMed
- Chien K.R. Stress pathways and heart failure. Cell. 1999;98:555–558. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous