Vascular permeability, vascular hyperpermeability and angiogenesis - PubMed (original) (raw)
Review
Vascular permeability, vascular hyperpermeability and angiogenesis
Janice A Nagy et al. Angiogenesis. 2008.
Abstract
The vascular system has the critical function of supplying tissues with nutrients and clearing waste products. To accomplish these goals, the vasculature must be sufficiently permeable to allow the free, bidirectional passage of small molecules and gases and, to a lesser extent, of plasma proteins. Physiologists and many vascular biologists differ as to the definition of vascular permeability and the proper methodology for its measurement. We review these conflicting views, finding that both provide useful but complementary information. Vascular permeability by any measure is dramatically increased in acute and chronic inflammation, cancer, and wound healing. This hyperpermeability is mediated by acute or chronic exposure to vascular permeabilizing agents, particularly vascular permeability factor/vascular endothelial growth factor (VPF/VEGF, VEGF-A). We demonstrate that three distinctly different types of vascular permeability can be distinguished, based on the different types of microvessels involved, the composition of the extravasate, and the anatomic pathways by which molecules of different size cross-vascular endothelium. These are the basal vascular permeability (BVP) of normal tissues, the acute vascular hyperpermeability (AVH) that occurs in response to a single, brief exposure to VEGF-A or other vascular permeabilizing agents, and the chronic vascular hyperpermeability (CVH) that characterizes pathological angiogenesis. Finally, we list the numerous (at least 25) gene products that different authors have found to affect vascular permeability in variously engineered mice and classify them with respect to their participation, as far as possible, in BVP, AVH and CVH. Further work will be required to elucidate the signaling pathways by which each of these molecules, and others likely to be discovered, mediate the different types of vascular permeability.
Figures
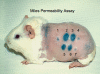
Fig. 1
Miles permeability assay. Various test substances were injected intradermally into the shaved and depilated flank skin of a Hartley guinea pig, followed immediately by an intravenous injection of Evan’s blue dye. Animal was photographed 30 min later. Injected materials were as follows: 1, Neutralizing antibody against VEGF-A; 2 and 5, ascites tumor-associated VEGF-A; 3 and 6, ascites tumor associated VEGF-A plus control immunoglobulin; 4 and 7, ascites tumor-associated VEGF-A plus specific VEGF-A neutralizing antibody. Reproduced from [93]
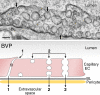
Fig. 2
Upper panel: Electron micrograph illustrating a typical capillary endothelial cell with numerous caveolae. Many of these are connected to the luminal or abluminal plasma membranes (arrows), whereas others are in the cytoplasm. L, lumen scale bar, 100 nm. Lower panel: Schematic diagram illustrating pathways by which molecules can cross the capillary barrier. (1) intercellular cleft; (2) caveolae that may shuttle across the capillary or form a chain of vesicles that connect the lumen and albumen. BL, basal lamina
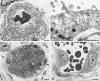
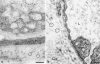
Fig. 3
Transmission electron micrographs of venules in normal mouse ear skin (a, b) and of a mother vessel (c, d) 3 days after local injection of Ad-VEGF-A164. (a, b) Typical normal venules lined by cuboidal endothelium. The cytoplasm contains prominent vesiculo-vacuolar organelles (VVOs) and is enveloped by a complete coating of pericytes (P). R, red blood cell. (c, d) MV are greatly enlarged vessels that are characterized by extensive endothelial cell thinning; striking reduction in VVOs and other cytoplasmic vesicles; prominent nuclei that project into the vascular lumen; frequent mitotic figures (arrows, c); endothelial cell bridging with the formation of multiple lumens (L, d); and pericyte (P) detachment in (c). The mother vessel lumen (c) is packed with red blood cells, indicative of extensive plasma extravasation. Inset. The normal venule depicted in a is reproduced in c at the same magnification as the mother vessel to illustrate differences in relative size of normal venules and MV. Scale bars: (a, b) 1 μm; (c, d) 5 μm
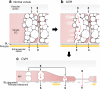
Fig. 4
(a) Schematic diagram of a normal venule comprised of cuboidal endothelium with prominent VVOs and closed inter-endothelial cell junctions. Note that some VVO vesicles attach to the intercellular cleft below the tight and adherens junction zones. 1 and 2 indicate potential pathways for transcellular (VVO) and intercellular (paracellular) plasma extravasation, respectively. Basal lamina (BL) is intact and the endothelium is completely covered by pericytes. (b) AVH. Acute exposure to VEGF-A causes VVO to open, allowing transcellular passage of plasma contents, possibly by mechanical pulling apart of stomatal diaphragms (3). Others have suggested that fluid extravasation takes place through an opening of intercellular junctions (4, here shown closed). BL and pericyte coverage are as in (a). (c) CVH. Prolonged VEGF-A stimulation causes venular endothelium to transform into MV, greatly thinned, hyperpermeable cells with fewer VVOs and VVO vesicles/vacuoles, degraded BL, and extensive loss of pericyte coverage. Plasma may extravasate either through residual VVO vesicles (5) or through fenestrae (6)
Fig. 5
Electron micrographs of portions of a MV (a) and a GMP (b) at 5 and 10 days following local injection of Ad-VEGF-A164 into nude mouse ears and 30 min after i.v. injection of ferritin tracer. (a) MV endothelium is greatly thinned and spanned by no more than 1–3 vesicles/vacuoles. Ferritin (dark-black particles) fill vascular lumens (L), are present in VVO vesicles/vacuoles, and have extravasated into the extravascular space (some encircled). (b) GMP with extensively thinned and fenestrated endothelium. Ferritin is present in lumen, occasional residual cytoplasmic vesicles (white arrows) and in extravascular space, some encircled. p, pericyte. Scale bars, 200 nm. Reprinted in revised form from [20]
Similar articles
- Orphan nuclear transcription factor TR3/Nur77 regulates microvessel permeability by targeting endothelial nitric oxide synthase and destabilizing endothelial junctions.
Zhao D, Qin L, Bourbon PM, James L, Dvorak HF, Zeng H. Zhao D, et al. Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):12066-71. doi: 10.1073/pnas.1018438108. Epub 2011 Jul 5. Proc Natl Acad Sci U S A. 2011. PMID: 21730126 Free PMC article. - Vascular hyperpermeability, angiogenesis, and stroma generation.
Nagy JA, Dvorak AM, Dvorak HF. Nagy JA, et al. Cold Spring Harb Perspect Med. 2012 Feb;2(2):a006544. doi: 10.1101/cshperspect.a006544. Cold Spring Harb Perspect Med. 2012. PMID: 22355795 Free PMC article. Review. - Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis.
Dvorak HF, Brown LF, Detmar M, Dvorak AM. Dvorak HF, et al. Am J Pathol. 1995 May;146(5):1029-39. Am J Pathol. 1995. PMID: 7538264 Free PMC article. Review. - Vascular permeability and pathological angiogenesis in caveolin-1-null mice.
Chang SH, Feng D, Nagy JA, Sciuto TE, Dvorak AM, Dvorak HF. Chang SH, et al. Am J Pathol. 2009 Oct;175(4):1768-76. doi: 10.2353/ajpath.2009.090171. Epub 2009 Sep 3. Am J Pathol. 2009. PMID: 19729487 Free PMC article. - Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine.
Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF. Brown LF, et al. EXS. 1997;79:233-69. doi: 10.1007/978-3-0348-9006-9_10. EXS. 1997. PMID: 9002222 Review.
Cited by
- Macromolecule extravasation-xenograft size matters: a systematic study using probe-based confocal laser endomicroscopy (pCLE).
Dietrich A, Stewart J, Huether M, Helm M, Schuetze C, Schnittler HJ, Jaffray DA, Kunz-Schughart LA. Dietrich A, et al. Mol Imaging Biol. 2013 Dec;15(6):693-702. doi: 10.1007/s11307-013-0641-z. Mol Imaging Biol. 2013. PMID: 23632953 Free PMC article. - Vascular assessment of wound healing: a clinical review.
Li WW, Carter MJ, Mashiach E, Guthrie SD. Li WW, et al. Int Wound J. 2017 Jun;14(3):460-469. doi: 10.1111/iwj.12622. Epub 2016 Jul 4. Int Wound J. 2017. PMID: 27374428 Free PMC article. Review. - Monomeric C-reactive protein and inflammation in age-related macular degeneration.
Chirco KR, Whitmore SS, Wang K, Potempa LA, Halder JA, Stone EM, Tucker BA, Mullins RF. Chirco KR, et al. J Pathol. 2016 Oct;240(2):173-83. doi: 10.1002/path.4766. Epub 2016 Sep 19. J Pathol. 2016. PMID: 27376713 Free PMC article. - 2-Desaza-annomontine (C81) impedes angiogenesis through reduced VEGFR2 expression derived from inhibition of CDC2-like kinases.
Zech TJ, Wolf A, Hector M, Bischoff-Kont I, Krishnathas GM, Kuntschar S, Schmid T, Bracher F, Langmann T, Fürst R. Zech TJ, et al. Angiogenesis. 2024 May;27(2):245-272. doi: 10.1007/s10456-024-09906-y. Epub 2024 Feb 26. Angiogenesis. 2024. PMID: 38403816 Free PMC article. - Dynamic contrast-enhanced magnetic resonance imaging for pretreatment prediction of early chemo-radiotherapy response in larynx and hypopharynx carcinoma.
Guo W, Luo D, Chen X, Lin M, Li L, Zhao Y, Yang L, Hu L, Zhao X, Zhou C. Guo W, et al. Oncotarget. 2017 May 16;8(20):33836-33843. doi: 10.18632/oncotarget.12952. Oncotarget. 2017. PMID: 27802182 Free PMC article.
References
- Dvorak H. Tumor blood vessels. New York: Aird, W. Cambridge University Press; 2007.
Publication types
MeSH terms
Grants and funding
- R01 HL071049/HL/NHLBI NIH HHS/United States
- R01 HL064402/HL/NHLBI NIH HHS/United States
- HL-64402/HL/NHLBI NIH HHS/United States
- R01 CA109086/CA/NCI NIH HHS/United States
- P01 CA92644/CA/NCI NIH HHS/United States
- CA 109086/CA/NCI NIH HHS/United States
- R01 CA050453/CA/NCI NIH HHS/United States
- P01 CA092644/CA/NCI NIH HHS/United States
- K01 CA098581/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources