Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by the p38 mitogen-activated protein kinase pathway - PubMed (original) (raw)
Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by the p38 mitogen-activated protein kinase pathway
Shinya Dohgu et al. Exp Neurol. 2008 Apr.
Abstract
Chronic systemic inflammation in the late stage of human immunodeficiency virus type-1 (HIV-1) infection could increase neuroinvasion of infected monocytes and cell-free virus, causing an aggravation of neurological disorders in AIDS patients. We previously showed that the peripheral administration of lipopolysaccharide (LPS) enhanced the uptake across the blood-brain barrier (BBB) of the HIV-1 viral protein gp120. Brain microvessel endothelial cells are targets of LPS. Here, we investigated whether the direct interaction between LPS and the BBB also affected HIV-1 transport using primary mouse brain microvessel endothelial cells (BMECs). LPS produced a dose (1-100 microg/mL)- and time (0.5-4 h)-dependent increase in HIV-1 transport and a decrease in transendothelial electrical resistance (TEER). Whereas indomethacin (cyclooxygenase inhibitor) and L-NAME (NO synthase inhibitor) did not affect the LPS-induced changes in HIV-1 transport or TEER, pentoxifylline (TNF-alpha inhibitor) attenuated the decrease in TEER induced by LPS, but not the LPS-induced increase in HIV-1 transport. LPS also increased the phosphorylation of p44/42 MAPK and p38 MAPK but not that of JNK. U0126 (p44/42 MAPK inhibitor) and SP600125 (JNK inhibitor) did not inhibit the LPS-induced increase in HIV-1 transport although U0126 attenuated the reduction in TEER. SB203580 (p38 MAPK inhibitor) inhibited the LPS-induced increase in HIV-1 transport without affecting TEER. Thus, LPS-enhanced HIV-1 transport is independent of changes in TEER and so is attributed to increased transcellular trafficking of HIV-1 across the BBB. These results show that LPS increases HIV-1 transcellular transport across the BBB by a pathway that is mediated by p38 MAPK phosphorylation in BMECs.
Figures
Figure 1
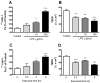
Effect of LPS on the permeability of BMECs to 131I-HIV-1 (A and C) and TEER (B and D). In panels A and B, BMECs were treated with LPS for 4 hr. In panels C and D, BMECs were treated with 100 μg/mL LPS. In panels A and C, results are expressed as % of control. Control values were 5.24 ± 0.71 × 10−5 and 1.75 ± 0.07 × 10−5 cm/min (A and C, respectively). Values are means ± SEM (n = 4–9). *P < 0.05, **P < 0.01, ***P < 0.001, significant differences from control.
Figure 2
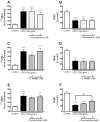
Effects of indomethacin, L-NAME, and pentoxifylline on the LPS-induced increase in permeability of 131I-HIV-1 to BMECs (A, C, and E) and the LPS-induced decrease in TEER (B, D, and F). BMECs were treated with LPS (100 μg/mL) for 4 hr in the presence or absence of indomethacin (1, 5 μM; A and B), L-NAME (1, 3 mM; C and D), or pentoxifylline (0.2, 2 mM; E and F). In panels A, C, and E, results are expressed as % of control. The control values of permeability coefficient for 131I-HIV-1 in panel A, C, and E were 2.90 ± 0.44 × 10−5, 2.83 ± 0.44 × 10−5, and 1.28 ± 0.09 × 10−5 cm/min, respectively. Values are means ± SEM (n = 6–21). *P < 0.05, **P < 0.01, ***P < 0.001, significant difference from control. #P < 0.05, significant difference from LPS (100 μg/mL).
Figure 3
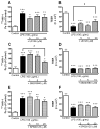
Effects of various MAPK inhibitors on the LPS-induced increase in permeability of 131I-HIV-1 to BMECs (A, C, and E) and the LPS-induced decrease in TEER (B, D, and F). BMECs were treated with LPS (100 μg/mL) for 4 hr in the presence or absence of U0126 (5–20 μM; A and B), SB203580 (1–10 μM; C and D), or SP600125 (5–20 μM; E and F). In panels A, C, and E, results are expressed as % of control. The control values of permeability coefficient for 131I-HIV-1 in panel A, C, and E were 1.47 ± 0.19 × 10−5, 2.04 ± 0.35 × 10−5, and 1.24 ± 0.91 × 10−5 cm/min, respectively. Values are means ± SEM (n = 3–18). *P < 0.05, **P < 0.01, ***P < 0.001, significant difference from control. #P < 0.05, significant difference from LPS (100 μg/mL).
Figure 4
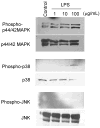
LPS increased phosphorylation of MAPKs in BMECs. BMECs were treated with LPS (1, 10 and 100 μg/mL) for 4 hr. Western blot analyses were performed to detect phosphorylated p44/42 MAPK, p38 MAPK and JNK as well as total p44/42 MAPK, p38 MAPK and JNK. Photographs are representative in three independent experiments.
Figure 5
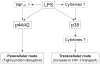
Schematic diagram of the mechanisms by which LPS induced the activation of MAPKs followed by the increase in the BBB permeability through paracellular and transcelluar routes. LPS-increased HIV-1 transport is mainly dependent on transcellular route. Dashed lines indicate possible intermediate steps.
Similar articles
- Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by luminal microvessel IL-6 and GM-CSF.
Dohgu S, Fleegal-DeMotta MA, Banks WA. Dohgu S, et al. J Neuroinflammation. 2011 Nov 30;8:167. doi: 10.1186/1742-2094-8-167. J Neuroinflammation. 2011. PMID: 22129063 Free PMC article. - A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers.
Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. Arrighi JF, et al. J Immunol. 2001 Mar 15;166(6):3837-45. doi: 10.4049/jimmunol.166.6.3837. J Immunol. 2001. PMID: 11238627 - The MAP kinase pathway mediates transcytosis induced by TNF-alpha in an in vitro blood-brain barrier model.
Miller F, Fenart L, Landry V, Coisne C, Cecchelli R, Dehouck MP, Buée-Scherrer V. Miller F, et al. Eur J Neurosci. 2005 Aug;22(4):835-44. doi: 10.1111/j.1460-9568.2005.04273.x. Eur J Neurosci. 2005. PMID: 16115207
Cited by
- Alpha Adrenergic Induction of Transport of Lysosomal Enzyme across the Blood-Brain Barrier.
Urayama A, Dohgu S, Robinson SM, Sly WS, Grubb JH, Banks WA. Urayama A, et al. PLoS One. 2015 Nov 6;10(11):e0142347. doi: 10.1371/journal.pone.0142347. eCollection 2015. PLoS One. 2015. PMID: 26545208 Free PMC article. - Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by luminal microvessel IL-6 and GM-CSF.
Dohgu S, Fleegal-DeMotta MA, Banks WA. Dohgu S, et al. J Neuroinflammation. 2011 Nov 30;8:167. doi: 10.1186/1742-2094-8-167. J Neuroinflammation. 2011. PMID: 22129063 Free PMC article. - Species-Dependent Blood-Brain Barrier Disruption of Lipopolysaccharide: Amelioration by Colistin In Vitro and In Vivo.
Jin L, Nation RL, Li J, Nicolazzo JA. Jin L, et al. Antimicrob Agents Chemother. 2013 Sep;57(9):4336-4342. doi: 10.1128/AAC.00765-13. Epub 2013 Jun 24. Antimicrob Agents Chemother. 2013. PMID: 23796941 Free PMC article. - Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications.
Hong S, Banks WA. Hong S, et al. Brain Behav Immun. 2015 Mar;45:1-12. doi: 10.1016/j.bbi.2014.10.008. Epub 2014 Oct 22. Brain Behav Immun. 2015. PMID: 25449672 Free PMC article. Review. - Lipopolysaccharide impairs blood-brain barrier P-glycoprotein function in mice through prostaglandin- and nitric oxide-independent pathways.
Salkeni MA, Lynch JL, Otamis-Price T, Banks WA. Salkeni MA, et al. J Neuroimmune Pharmacol. 2009 Jun;4(2):276-82. doi: 10.1007/s11481-008-9138-y. Epub 2008 Nov 28. J Neuroimmune Pharmacol. 2009. PMID: 19039663 Free PMC article.
References
- Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. - PubMed
- Alonso K, Pontiggia P, Medenica R, Rizzo S. Cytokine patterns in adults with AIDS. Immunol Invest. 1997;26:341–350. - PubMed
- András IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003;74:255–265. - PubMed
- András IE, Pu H, Tian J, Deli MA, Nath A, Hennig B, Toborek M. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab. 2005;25:1159–1170. - PubMed
- Banks WA, Akerstrom V, Kastin AJ. Adsorptive endocytosis mediates the passage of HIV-1 across the blood-brain barrier: evidence for a post-internalization coreceptor. J Cell Sci. 1998;111:533–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS050547/NS/NINDS NIH HHS/United States
- R01 NS050547/NS/NINDS NIH HHS/United States
- R01 NS050547-03/NS/NINDS NIH HHS/United States
- R01 AG029839/AG/NIA NIH HHS/United States
- N01CO12400/CA/NCI NIH HHS/United States
- N01-CO-12400/CO/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials